On-Chip PCR Based Plasmonic Microfluidic Platform: Ultrafast Point-of-Care Diagnostics of SARS-CoV-2
Dr. Megha Agrawal*
Chief Editor
Biotechnology Kiosk, USA
Abstract
Keywords: PCR, Plasmonics, SARS-CoV-2, Molecular Diagnostics, Pandemic
To cite this article: Agrawal M, On-Chip PCR Based Plasmonic Microfluidic Platform: Ultrafast Point-of-Care Diagnostics of SARS-CoV-2, Biotechnol. kiosk, Vol 3, Issue 1, PP: 5-11 (2021); DOI: https://doi.org/10.37756/bk.21.3.1.1
*E-mail: meghaagra@gmail.com; megha@biotechkiosk.com
- Introduction
It is widely recognized that rapid diagnosis of COVID-19 and other highly contagious viral diseases is essential to ensure timely medical care, quarantining and contact tracing. Reverse transcription-polymerase chain reaction (RT-PCR) is considered the gold standard worldwide for diagnosis during the COVID-19 pandemic. RT-PCR normally uses enzymes to reverse transcribe tiny amounts of viral RNA to DNA, which is then used to amplify the DNA. This allows detection by a fluorescent probe. RT-PCR is proven to be the most sensitive and reliable diagnostic method. However, bulky and expensive machines are required to complete the PCR portion of the test. Further, the conventional real-time PCR thermal cyclers are based on the Peltier effect that have a long turnaround time of PCR for more than 1 hour. The reason of the lengthy time to complete the PCR portion of the test is due to the fact that it requires 30-40 cycles of heating and cooling in special machines. Moreover, the sample preparation along with false negative results can extend the diagnostic waiting time in multiple PCR tests. Additionally, samples are usually sent out to a laboratory that causes further delays as the patients are required to wait a day or two to receive their diagnosis results. All these procedural and logistic difficulties suggest that time-consuming, bulky, and expensive equipment and analytical steps for real-time PCR based diagnostics have serious limitations that prevent for immediate infection diagnostics at point-of-care (POC) level [1-5].
Researchers have been actively engaged in the development of PCR cyclers for POC diagnostics that have been demonstrated for accurate and fast thermal cycling to increase heating ramp. To this end, photonic PCR utilizing plasmonic materials has been applied that has shown potentials for substantially reducing the amplification time as a result of the ultrafast and noncontact light-to-heat conversion. In plasmonic principles based designs, gold nanospheres, nanorods, bipyramids, or nanoshell dispersed in PCR solution have been employed that have been shown to accelerate photothermal heating under laser irradiation. In these systems, light absorption of gold nanoparticles (AuNPs) excites hot electrons that result in strong non-radiative relaxation within tens of femtoseconds. This subsequently produces uniform and volumetric heat of sample solution. Current research has focused on addressing some technical challenges such as temperature gradient, PCR efficiency, and real-time monitoring that are needed to be overcome for rapid and quantitative molecular diagnostics [6-8].
On-chip PCRs are considered one of the best technology options for fast and reliable POC diagnostics [9]. It is believed that such a technology can be leveraged for the miniaturization and integration of POC testing systems. Especially, low thermal capacities are possible to obtain due to small sample volume and high surface-to-volume ratio, which results in fast heating/cooling rates and short reaction time [9]. The advantage is that the technology utilizes spatial and temporal thermal cycling that allows continuous or droplet sample fluid flows along a microfluidic channel with spatially discrete temperature regions for PCR cycling by using different resistive microheaters. Further, a small volume of sample solution in microchambers can perform rapid PCR using time-variant thermal cycling of Peltier heater, thin film heater, and infrared laser heater. To make higher-performance on-chip PCRs for practical POC applications, current considerations are to overcome technical challenges such as external pumping or microbubble formation [9].
- Nanoplasmonic On-Chip PCR: Rapid and Quantitative Molecular Diagnostics
Recently, researchers demonstrated ultrafast and real-time nanoplasmonic on-chip PCR for rapid and quantitative molecular diagnostics using nanoplasmonics and vacuum-assisted microfluidics (Figure 1) [9]. The so-called plasmofluidic PCR (PF-PCR) chip allowed vacuum-assisted easy sample loading into dead-end reaction microchamber arrays that were enclosed by pre-charged vacuum cell and vapor barrier (Figure 1). They employed vacuum cell with a high gas-permeability layer (HPL) on the wall of microchamber arrays that effectively induced spontaneous loading of sample solution and continuous removal of microbubbles trapped or newly produced during the reaction [9].
In this study, the vapor barrier was shown to have a low gas-permeability layer (LPL) that helped the prevention of solution evaporation during the reaction cycles of high temperature. Glass nanopillar arrays with Au nanoislands were then employed that produced ultrafast nanoplasmonic heating resulting from strong light-to-heat conversion of white light emitting diode ‘WLED’ as well as rapid cooling due to a large surface-to-volume ratio of nanopillars. This PF-PCR chip provided rapid and spontaneous sample loading, ultrafast thermal cycling, and real-time quantification with bubble-free environment for quantitative POC molecular diagnostic applications [9].
In this design of the on-chip plasmonic PCR, a postage stamp-sized polydimethylsiloxane chip was employed that had a microchamber array for the PCR reactions. Upon adding a drop of sample to the chip, the liquid was pulled into the microchambers by the applied vacuum. It was then positioned above glass nanopillars with gold nanoislands. Further, microbubbles that could interfere with the PCR reaction, diffused out through an air-permeable wall. Subsequently, by turning a white LED on beneath the chip, the gold nanoislands on the nanopillars quickly converted light to heat that rapidly cooled when the light was turned off (Figure 1) [9].

- On-Chip PCR for the Rapid Diagnostics of SARS-CoV-2
Researchers demonstrated the nanoplasmonic on-chip PCR for ultrafast amplification and in-situ cyclic real-time quantification of lambda DNA and E gene as a target for SARS-CoV-2 by alternating the WLED illumination and the fluorescence detection. In this process, nanoplasmonic photothermal modulation of the NPS allowed rapid and efficient thermal cycling inside microfluidic channels of the PF-PCR chip. Further, the researchers tested the device on a piece of DNA containing a SARS-CoV-2 gene, accomplishing 40 heating and cooling cycles and fluorescence detection in only 5 minutes, with an additional 3 minutes for sample loading (Figure 2) [9]. They showed that by adding a step of the reverse transcriptase prior to sample loading, the entire testing time with the new method was drastically reduced to 10-13 minutes compared to about an hour for typical RT-PCR testing [9].
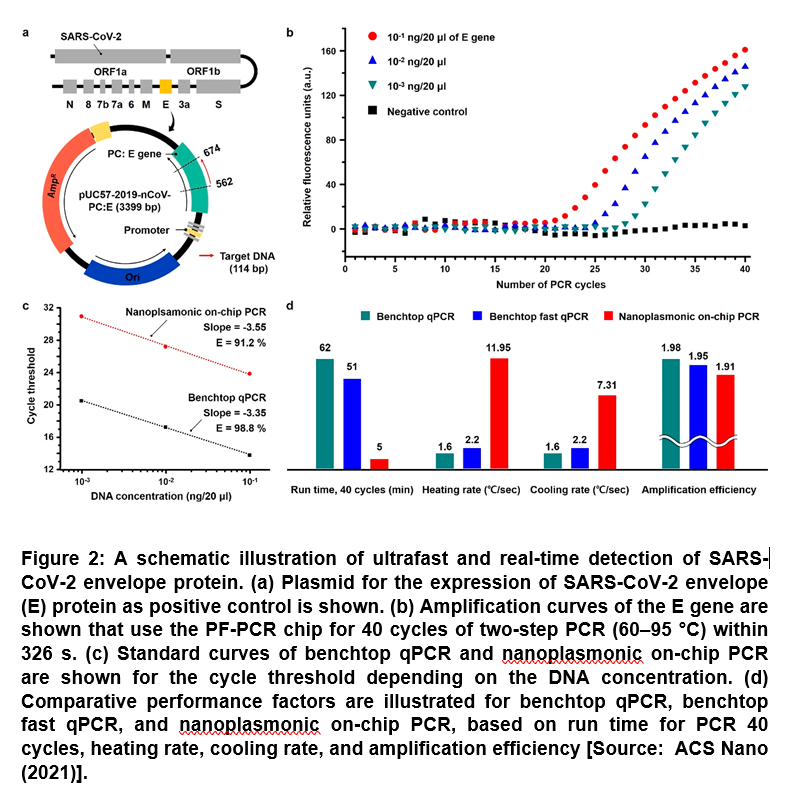
It was shown that both the nanoplasmonic heating and the thin sample confinement resulted in uniform vertical distribution of photothermal heat in a small volume for rapid and efficient thermal cycling. The vacuum-enabled PF-PCR chip of bilayered PDMS configuration not only allowed spontaneous and rapid sample loading without trapped microbubbles but it also helped maintain stable reaction during the full PCR cycles [9]. The real-time on-chip PCR was shown to exhibit the ultrafast 40 cycle amplification of λ-DNA for 264 s and SARS-CoV-2 for 306 s as well as the in-situ quantification of amplicons with high efficiency over 91% [9]. The new device could pave the way to many opportunities for rapid POC diagnostics during the pandemic [9].
- Concluding Remarks
The latest advances in rapid diagnostics of COVID-19 offer new frontiers to mitigate the challenges posed by the pandemic. Especially, the technology that involves on-chip PCR for POC diagnostics is anticipated to impact the field of diagnostics of highly contagious viral diseases in the near future.
References
[1] Kim, M. N.; Ko, Y. J.; Seong, M. W.; Kim, J. S.; Shin, B. M.; Sung, H. Analytical and Clinical Validation of Six Commercial Middle East Respiratory Syndrome Coronavirus RNA Detection Kits Based on Real-Time Reverse-Transcription PCR. Ann. Lab. Med. 2016, 36, 450– 456, DOI: https://doi.og/10.3343/alm.2016.36.5.450.
[2] Nguyen, T.; Duong Bang, D.; Wolff, A. 2019 Novel Coronavirus Disease (COVID-19): Paving the Road for Rapid Detection and Point-of-Care Diagnostics. Micromachines-Basel 2020, 11, 306, DOI: https://doi.org/10.3390/mi11030306.
[3] Du, Z. W.; Wang, L.; Cauchemez, S.; Xu, X. K.; Wang, X. W.; Cowling, B. J.; Meyers, L. A. Risk for Transportation of Coronavirus Disease from Wuhan to Other Cities in China. Emerging Infect. Dis. 2020, 26, 1049– 1052, DOI: https://doi.org/10.3201/eid2605.200146.
[4] Corman, V. M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D. K. W.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M. L.; Mulders, D. G. J. C.; Haagmans, B. L.; van der Veer, B.; van den Brink, S.; Wijsman, L.; Goderski, G.; Romette, J. L.; Ellis, J.; Zambon, M.; Peiris, M.Detection of 2019 Novel Coronavirus (2019-nCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 23– 30, DOI: https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
[5] Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; Yao, H.; Li, N.; Zhao, H.; Feng, Y.; Liu, S.; Zhang, Q.; Liu, D.; Yuan, J. Rapid and Visual Detection of 2019 Novel Coronavirus (SARS-CoV-2) by a Reverse Transcription Loop-Mediated Isothermal Amplification Assay. Clin. Microbiol. Infect. 2020, 26, 773– 779, DOI: https://doi.org/10.1016/j.cmi.2020.04.001.
[6] You, M.; Li, Z.; Feng, S.; Gao, B.; Yao, C.; Hu, J.; Xu, F. Ultrafast Photonic PCR Based on Photothermal Nanomaterials. Trends Biotechnol. 2020, 38, 637– 649, DOI: https://doi.org/10.1016/j.tibtech.2019.12.006.
[7] You, M.; Cao, L.; Xu, F. Plasmon-Driven Ultrafast Photonic PCR. Trends Biochem. Sci. 2020, 45, 174– 175, DOI: https://doi.org/10.1016/j.tibs.2019.11.007.
[8] Lee, Y.; Kang, B. H.; Kang, M.; Chung, D. R.; Yi, G. S.; Lee, L. P.; Jeong, K. H. Nanoplasmonic On-Chip PCR for Rapid Precision Molecular Diagnostics. ACS Appl. Mater. Interfaces 2020, 12, 12533– 12540,
DOI: https://doi.org/10.1021/acsami.9b23591.
[9] Byoung-Hoon Kang, Youngseop Lee, Eun-Sil Yu, Hamin Na, Minhee Kang, Hee Jae Huh and Ki-Hun Jeong, Ultrafast and Real-Time Nanoplasmonic On-Chip Polymerase Chain Reaction for Rapid and Quantitative Molecular Diagnostics, ACS Nano 2021, DOI: https://doi.org/10.1021/acsnano.1c02154.