
Lately, cardio regenerative therapeutic approaches in clinic have received strong boost from promising research advances in stem cells. Researchers have shown applications of the first generation of clinical trials using cell-based therapies in the heart that have been performed with bone marrow and adipose tissue derived mesenchymal stem cellsshortcomings in the first generation cells. Second generation cell therapies are considered superior and being considered towards the use of cardiac-committed cell populations. These include cardiac progenitor cells and pluripotent stem cell derived cardiomyocytes. However, translating the research laboratory results along with pre-clinical data into effective clinical treatments is still thought to be challenging. This is due to the existing lack of knowledge on the regenerative mechanisms of action of these therapeutic products in addition to the stringent regulatory and safety concerns. This has prompted researchers to consider advanced analytical methods for characterization of such complex products and a deep understanding of their therapeutic effects at the cell and molecular level. This characterization is considered very critical to overcome the challenges and make these cells compatible with the advanced therapies.To this end, omics technologies including proteomics and glyco(proteo)mics that are based on state-of-the-art mass-spectrometry have shown tremendous promise to generate novel data on cell biology along with the required assessment of cell based-products that are applied in cardiac regeneration strategies. In this perspective, we have described proteomics and glycoproteomics and discuss the impact of omics technologies on cardiac progenitor cells and pluripotent stem cell derived cardiomyocytes biology cardiac regenerative therapies.
Key words: Cardiac repair, proteomics, glycoproteomics, stem cells, regenerative therapies.
*E-mail: pallavi.tripathi@manchester.ac.uk
Introduction
It is estimated that life expectancy worldwide will continue to increase in the upcoming decades, which may lead to a higher prevalence of age-related diseases such as cancer, neurodegenerative disorders and cardiovascular diseases. Among all these diseases, Acute Myocardial Infarction (AMI), which belongs to one of the severe cardiovascular medical conditions is considered lethal and does not give much time to recover. AMI or heart attack is known to cause millions of deaths worldwide each year. AMI causes serious damage to the patient’s health as it results in irreversible myocardial tissue damage with loss of cardiomyocytes (CMs) that are the main cell type in the heart. Human body responds to this ischemic attack and the affected area becomes fibrotic with accumulation of extracellular matrix proteins to compensate the damage. Studies conducted on the fibrotic tissue have revealed that such tissue is stiffer with different electromechanical properties in comparison to healthy myocardium. Consequently, this leads to impaired cardiac output. To overcome this medical problem, several therapeutic strategies have been suggested that include medications, such as beta-blockers and angiotensin converting enzyme inhibitors. While these therapies have proven to be successful in reducing mortality, they do not allow for the complete restoration of normal heart muscle function. This includes preventing progression to chronic heart failure that gives rise to with high mortality rates. The only available treatment in such scenario is heart transplantation or the use of mechanical circulatory assist devices [1, 2].
The recent concept of Advanced Therapy Medicinal Products (ATMPs) for cardio regenerative medicine has emerged due to the increasing incidents of heart failure and the scarcity of donor organs for heart transplantation. ATMPs are considered promising for heart disease treatments that include cell transplantation-based strategies with cardiac progenitor cells (CPCs) or pluripotent stem cell derived cardiomyocytes (PSC-CMs). These treatment methodologies have been used in a number of pre-clinical and clinical trials. Further, it has been shown that extracellular vesicles through biomaterial-based delivery systems can also preserve cardiac tissue after myocardial infarction [1]. Especially, studies have suggested that exosomal microRNAs can play a key role in cardiac regeneration. Despite these advances, there are still challenges involving preclinical studies and clinical trials that do not show consistent physiological improvements and benefits over standard pharmacological treatment procedures. In other words, the goal is to translate the potential of stem cells and other ATMPs pre-clinical data into effective treatments that are used in the clinical set. To achieve this goal, there is a need for analytical methods that permit a deep characterization of such complex ATMPs along with determining their potency and the assessment of their mechanisms of action in humans [3, 4].
Recent advances in omics technologies have fulfilled the demand of accurate and reliable analytical techniques and software. These technologies have allowed comprehensive and non-targeted investigation of these cell populations, which enable accomplishing a more complete molecular characterization and reveal key mechanisms of action. The most well-known omics technologies are proteomics and glyco(proteo)mics based tools. The workflows of these omics tools are based on state of the art mass-spectrometry that have paved way to some of the major breakthroughs in cell biology and a detailed assessment of cell based-products applied in cardiac regeneration strategies. These omics approaches are focused on the profiling of protein and glycan signatures for identification and characterization of cell populations. This can potentially lead to the discovery of pluripotency and differentiation biomarkers along with providing new insight into paracrine mechanisms and signaling cascades that are involved in cardiac repair. It is believed that the gained knowledge from the omics-based characterization and analysis would pave the way to a more rational therapy design in ATMPs [1].
In this short perspective, we have discussed recent trends and applications of omics technologies in cardiac repair.
Cell-Based Therapies for Cardiac Repair
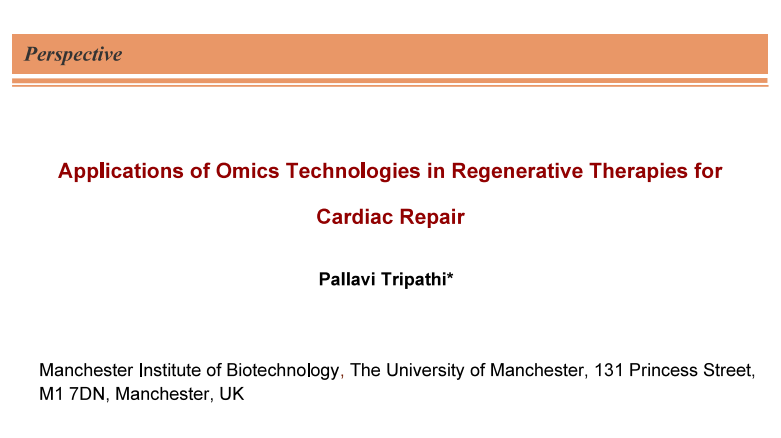
Researchers employed multiple cell types in several pre-clinical studies and clinical trials (Figure 1). These approaches were mostly based on cells including skeletal myoblasts, mesenchymal stem cells, bone marrow mononuclear cells, and blood-circulating progenitors [1]. While, several studies showed most of these first-generation cell-based therapies to be safe, there were some data that showed poor clinical effects. Researchers attributed the failures of these therapies to the cell heterogeneity, lack of cell engraftment, and also inability of cells to differentiate and replace cardiac tissue. To overcome these challenges, current research has focused on second-generation cell-based therapies that include CPCs and PSC-CMs, which give more purified, homogenous and cardiac-committed cell populations [5-9]. To this end, several in-vitro and preclinical studies have been conducted that have shown activation of different populations of CPCs upon AMI. It has been shown that these second generation cells proliferate and migrate to the site of injury. Subsequently, these cells release important paracrine factors and microvesicles that are involved in angiogenesis, vasculogenesis, immunomodulation and CM cytoprotection [10-12].
Researchers have also demonstrated injection of human CPCs (hCPCs) in animal models of AMI that have resulted in an overall reduction of scar tissue size and improvements in heart function. In these studies, preclinical meta-analysis has been done that has shown an estimated improvement of 10.7% of heart left ejection fraction [1]. These promising preclinical results have initiated growth in research and developments in rapid translation of laboratory research into the clinic. This has been achieved with different populations of hCPCs in clinical trials that consist of both in autologous and allogeneic settings. To this end, pluripotent stem cells (PSCs) that include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have shown tremendous promise in proliferative potential. This also include the capacity to differentiate into all cells from the three primary germ layers involving mesoderm, ectoderm and endoderm and CMs. Studies have clearly indicated ability of PSCs to theoretically provide an unlimited number of CMs that has made PSC-CMs. This is potentially very exciting as a novel clinical option for the replacement of the endogenous CMs that are lost in AMI and chronic heart failure patients (Figure 1) [1, 13-16]. However, analytical characterization is the major issue/requirement that we will discuss next.
Omics Technologies for Characterization of Cells
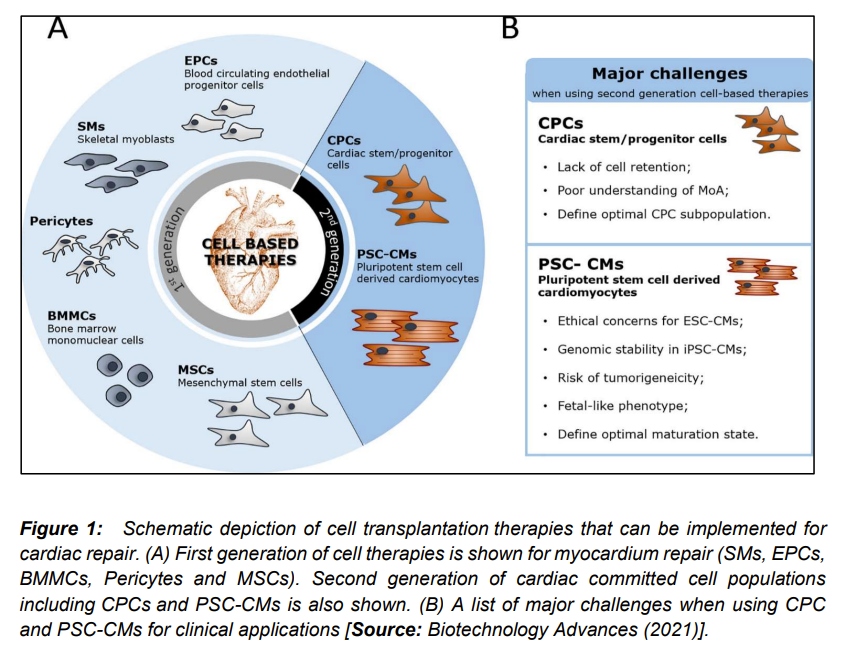
Several studies have been conducted using different omics methodologies (Figure 2) to the characterization of CPC and PSC-CMs [1]. These studies include gene expression profile employing transcriptomics, epigenomic modifications by epigenomics, analysis of nutrients and other small molecules that arise from the cell’s energy metabolism by metabolomics, proteins using proteomics and protein post translational modifications by employing glycomics. Researchers have also employed complex multi-omics approaches for analyzing different datasets and their interactions. These different types of omics platforms are known to generate big amounts of data. These datasets are analyzed by using different omics methodologies that include bioinformatic processing tool and deconvolute and perform functional annotation of experimental data [17-21].
Researchers have shown Single Proteomics by MS (SCoPE-MS) for quantification of thousands of proteins in single cell samples. This has enabled to measure proteome fingerprints of single cells and linking them to different phenotypes. On the other hand, glycoproteomics is another form of proteomics, which is focused on identifying glycosylated proteins and mapping their sites of glycosylation. This helps to gain a better understanding of the function of glycoproteins in biological or disease states. One of the applications of glycoproteomics is in genome-editing technologies that has been shown to reduce glycosylation complexity to facilitate glycoproteomic analysis [1, 22].
It is believed that progress of cell transplantation and cell-free clinical therapies for heart regeneration will be substantially moved forward by the applications of proteomics and glyco(proteo)mics methodologies. These omics technologies are supposed to assist in translation of these therapeutic approaches to practical applications, for example in novel biomarkers of heart disease (Figure 2) [1]. In addition, studies based on proteomics and glyco(proteo)mic are considered to play key roles in developing strategies to better characterize and interrogate PSC-CMs and CPCs populations. This is expected to reveal important insights that can help to elucidate their membrane molecular landscape, molecular processes regulating cardiac differentiation. This also include integration into the target tissue, and regenerative molecular mechanisms of those cells and corresponding cell-derived products [1, 23-25].
Conclusion and Outlook
It is believed that applications of omics analytics technologies will help advance analytical assays to identify stem cell population subtypes. This can also help understand the regenerative mechanisms of action of stem cells and their impact on the cardiac tissue. This understanding is critical because it can enable researchers to better define the quality requirements for an accelerated clinical translation of stem-cell based products in cardiac repair. With further developments, proteomic and glyco(proteo)mic profiling strategies are supposed to become more sensitive, accurate and high throughput methodologies. This is expected to pave the way to advanced characterization of stem cells and stem cell derived products in homeostasis and disease settings. To this end, researchers have demonstrated potential of untargeted high-throughput proteomic and glyco(proteo)mic characterization of cardiac populations and cardiac cell derived products. Studies have been conducted to reveal key molecular pathways and proteomic/glycan signatures that leverage strategies for differentiation, purification and application of CPCs, PSC-CMs and/or cell derived products in clinical applications. Future studies are anticipated to focus on a better definition of critical quality attributes of these cells and cell-based products. This is believed to lead to future regulatory frameworks that could accelerate the clinical applications of stem cell based cardiac repair and therapies based on omics methodologies.
References
[1] Sebastião, M. J., Marcos-Silva, L.; Gomes-Alves, P.; M. Alves, P. Proteomic and Glyco(proteo)mic tools in the profiling of cardiac progenitors and pluripotent stem cell derived cardiomyocytes: Accelerating translation into therapy. Biotechnology Advances 2021, 49,107755, doi: https://doi.org/10.1016/j.biotechadv.2021.107755.
[2] Benjamin, E. J.; Blaha, M. J.; Chiuve, S. E.; Cushman, M.; et. al. American Heart Association Statistics Committee, Stroke Statistics Subcommittee, Heart disease and stroke statistics—2017 update: a report from the American Heart Association, Circulation, 2017, 135, doi: https://doi.org/10.1161/CIR.0000000000000485.
[3] Luo, L.; Li, T.-S. Mini review: Recent advances in the cell-based therapies for cardiac regeneration. Curr. Stem Cell Res Ther 2020, 15(8), 649-660, doi: https://doi.org/10.2174/1574888×15666200102103755.
[4] de Abreu, R. C.; Fernandes, H.; P.A. da Costa, M.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol 2020, 17, 685-697, doi: https://doi.org/10.1038/s41569-020-0389-5.
[5] Menasché, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L. et. al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial. Circulation 2008, 117, 1189-1200, doi: https://doi.org/10.1161/CIRCULATIONAHA.107.734103.
[6] Cambria, E.; Pasqualini, F. S.; Wolint, P.; Günter, J.; Steiger, J.; Bopp ,A.; Hoerstrup, S. P.; Emmert, M. Y. Translational cardiac stem cell therapy: advancing from first-generation to next-generation cell types. NPJ Regen. Med 2017, 2, 17, doi: https://doi.org/10.1038/s41536-017-0024-1.
[7] Alvino, V. V.; Fernández-Jiménez, R. Rodriguez-Arabaolaza, I.; Slater, S.; Mangialardi, G.; Avolio, E.; Spencer, H.; Culliford, L.; Hassan, S.; Ballesteros, L. S.; et. al. Transplantation of allogeneic pericytes improves myocardial vascularization and reduces interstitial fibrosis in a swine model of reperfused acute myocardial infarction. J. Am. Heart Assoc 2018, 7(2), e006727, doi: https://doi.org/10.1161/JAHA.117.006727.
[8] Avolio, E. et. a. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ. Res 2015, 116, e81-e94, doi: https://doi.org/10.1161/CIRCRESAHA.115.306146.
[9] Crisostomo, V.; Casado, J. G.; Baez-Diaz, Blazquez, C. R.; Sanchez-Margallo F. M. Allogeneic cardiac stem cell administration for acute myocardial infarction.
Expert. Rev., Cardiovasc. Ther 2015, 13, 285-299, doi: https://doi.org/10.1586/14779072.2015.1011621.
[10] Sebastião,Maria J. R. et. al. Human cardiac stem cells inhibit lymphocyte proliferation through paracrine mechanisms that correlate with indoleamine 2,3-dioxygenase induction and activity. Stem Cell Res Ther 2018, 9, 290, doi: https://doi.org/10.1186/s13287-018-1010-2.
[11] Sebastião, M. J.; Serra, M.; Pereira, R.; Palacios, I.; Gomes-Alves, P.; Alves P. M. Human cardiac progenitor cell activation and regeneration mechanisms: exploring a novel myocardial ischemia/reperfusion in vitro model. Stem Cell Res Ther 2019, 10, 77, doi: https://doi.org/10.1186/s13287-019-1174-4.
[12] Sharma, S. et. al. Cardiosphere-derived cells from pediatric end-stage heart failure patients have enhanced functional activity due to the heat shock response regulating the secretome. Stem Cells, 2015, 33, 1213-1229, doi: https://doi.org/10.1002/stem.1937.
[13] Zwetsloot, P. P. et. al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ. Res. 2016, 118 ,1223-1232, doi: https://doi.org/10.1161/CIRCRESAHA.115.307676.
[14] Funakoshi, S.; Miki, K. et. al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep 2016, 6, 19111, doi: https://doi.org/10.1038/srep19111.
[15] Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y. et. al.
Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388-391, doi: https://doi.org/10.1038/nature19815.
[16] Liu, Y.-W. et. al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol 2018, 36, 597-605, doi: https://doi.org/10.1038/nbt.4162.
[17] Cyganek, L.et. al. Deep phenotyping of human induced pluripotent stem cell–derived atrial and ventricular cardiomyocytes. JCI Insight 2018, 3(12), e99941.doi: https://doi.org/10.1172/jci.insight.99941.
[18] Fujita, J.; Tohyama, S.; Kishino, Y.; Okada, M.; Morita, Y.
Concise review: genetic and epigenetic regulation of cardiac differentiation from human pluripotent stem cells. Stem Cells 2019, 37,992–1002, doi: https://doi.org/10.1002/stem.3027.
[19] Zhang, Y.; Zhong, J. F.; Qiu, H.; MacLellan, W. R.; Marbán, E.; Wang, C.
Epigenomic reprogramming of adult Cardiomyocyte-derived cardiac progenitor cells, Sci. Rep 2015, 5, 17686, doi://doi.org/10.1038/srep17686.
[20] Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Teixeira, A.; Domian, I.; Serra, M.;
Alves, P. M. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep 2017, 7, 8590, doi: https://doi.org/10.1038/s41598-017-08713-4.
[21] Joshi, A.; Rienks, M.; Theofilatos, K.; Mayr, M. Systems biology in cardiovascular disease: a multiomics approach, Nat. Rev. Cardiol. 2021, 18, 313–330, doi: https://doi.org/10.1038/s41569-020-00477-1.
[22] Slavov, N. Unpicking the proteome in single cells, Science 2020, 80, 367(6477), 512-513, doi: https://doi.org/10.1126/science.aaz6695.
[23] Christensen, G.; Herum, K. M.; Lunde, I. G. Sweet, yet underappreciated: proteoglycans and extracellular matrix remodeling in heart disease.Matrix Biol. 2019, 75(76), 286-299, doi: https://doi.org/10.1016/j.matbio.2018.01.001.
[24] Israr, M. Z.; Heaney, L. M.; Suzuki, T. Proteomic biomarkers of heart failure. Heart Fail. Clin. 2018, 14(1), 93-107, doi: https://doi.org/10.1016/j.hfc.2017.08.010.
[25] Fu, Q.; Van Eyk, J. E. Proteomics and heart disease: identifying biomarkers of clinical utility, Expert Rev. Proteomics 2006, 3(2), 237-249, doi: https://doi.org/10.1586/14789450.3.2.237.