Abstract1 Vol2 issue6
July 20, 2020Abstract2 Vol2 Issue6
July 20, 2020
Abstract
SARS-CoV-2, the highly transmissible and infectious novel coronavirus, is responsible for the current COVID-19 pandemic. Therapeutic efforts toward the development of a vaccine and other treatment modalities are a priority. High-affinity neutralizing antibodies play a critical role in protection against viruses so a rapid development to identify such neutralizers against SARS-CoV-2 that also cross-protect against other coronaviruses is warranted. Here, we review current research on the isolation of SARS-CoV-2 neutralizing monoclonal antibodies from B cells of convalescent patients and their effectiveness in a small animal model to limit the viral disease. The antibodies that were able to successfully treat COVID-19 in the animals were highly specific against the ACE2 binding site of the viral spike protein and thus efficiently blocked infection.
Keywords: SARS-CoV-2, COVID-19, nAbs, ACE2, Spike protein, monoclonal antibody, antibody therapy
To cite this article: Chakraborty S; Neutralizing antibodies: Viable treatment modality for COVID-19, Biotechnology Kiosk, Vol 2, Issue 6, PP: 10-16 (2020); DOI: https://doi.org/10.37756/bk.20.2.6.1
Introduction
COVID-19 (coronavirus disease 2019), caused by SARS-CoV-2 has created quite a mayhem in the public health domain within a short span of time. With more than 400,000 deaths worldwide as of 19th June 2020, the race is on to create a vaccine against the virus. In the interim, other therapeutic efforts to prevent or limit the disease have also gained momentum. Researchers are focusing on old antiviral drugs and antibody-based therapies to curb the spread of SARS-CoV-2.
Repurposed old drugs like remdesivir—a broad spectrum antiviral, baricitinib—an oral JAK1/JAK2 inhibitor and dexamethasone—a glucocorticoid have shown promising results in late-stage clinical trials while Ly-CoV555—a neutralizing monoclonal antibody against SARS-CoV-2 spike (S) protein has already entered phase I trials as the world’s first potential COVID-19 antibody treatment. One of the critical advantages of neutralizing antibodies is their specificity toward the viral target that selectively and directly blocks infection. But beyond just blunting the viral propagation, these antibodies have the potential to generate vaccine-like effects also by inducing or modulating the endogenous antiviral immune responses (eliciting both humoral and cellular responses)[1]. Unlike cancer and inflammatory disease, where antibodies constitute a large proportion of treatment modalities, their use against viral infections has not gained much popularity until recently in the COVID-19 era.
In light of the lack of effective therapies for COVID-19, usage of convalescent plasma with high neutralizing titers has been shown to provide some clinical benefits to hospitalized patients. Given the limitations associated with such therapies, including variability in titers, contamination risks, and circulatory overload, numerous researchers have focused on employing monoclonal antibodies instead [2] .
Antibody Therapy against viruses
Monoclonal antibodies (mAbs) have shown enormous potential in the last two decades as a significant therapeutic modality against infectious diseases, especially with Ebola and HIV [3]. Antiviral mAbs can limit viral propagation in a number of ways a) by physically blocking the site of virus interaction with host cell—also known as neutralization b) by coating the virus in an immune complex to make it susceptible to digestion by serum complement or professional phagocytes, and c) by facilitating endocytosis of antibody bound virions into professional antigen-presenting cells (APCs) leading to an antiviral cellular immune response [4].
Enveloped viruses like SARS-CoV-2 usually elicit strong antibody responses against their envelope spike proteins that lay exposed on the surface [1]. The repertoire of such antibodies against spike proteins may reduce viral burden through several mechanisms, but only a subset of those can neutralize the infectivity of the virus to host cells. In other words, not all elicited antibodies that bind to the spike protein can neutralize the virus [5]. Therefore, for therapeutic use, isolation of neutralizing monoclonal antibodies (nAbs) that can bind (with high affinity) to the host cell interacting sites of the spike protein and block the biological activity/infectivity of the virus, is highly desirable. Monoclonal nAbs are isolated from single-memory or plasma B cells retrieved from vaccinated or naturally infected animals and human donors. A nAb against the respiratory syncytial virus (RSV) is in clinical use to prophylactically prevent infections in infants [6].
The case of SARS-CoV-2
Coronaviruses are enveloped viruses, and as described above, the viral surface spike (S) protein presents a predominant target for nAbs. SARS-CoV-2 S protein—a trimeric type I membrane glycoprotein—mediates viral attachment and entry into host cells through binding to the ACE2 receptor. The approximately 200kDa S protein has two functional subunits—S1 (further divided into four core domains S1A through S1D) and S2, based on a host protease cleave site. The receptor-binding domain (RBD) resides in the N-terminal S1 subunit, while membrane-anchored S2 facilitates the fusion of the viral membrane with cellular membrane. Neutralizing antibodies against the RBD that can disable receptor interactions are often the most potent neutralizers, desired for their therapeutic potential [7].[Figure 1].
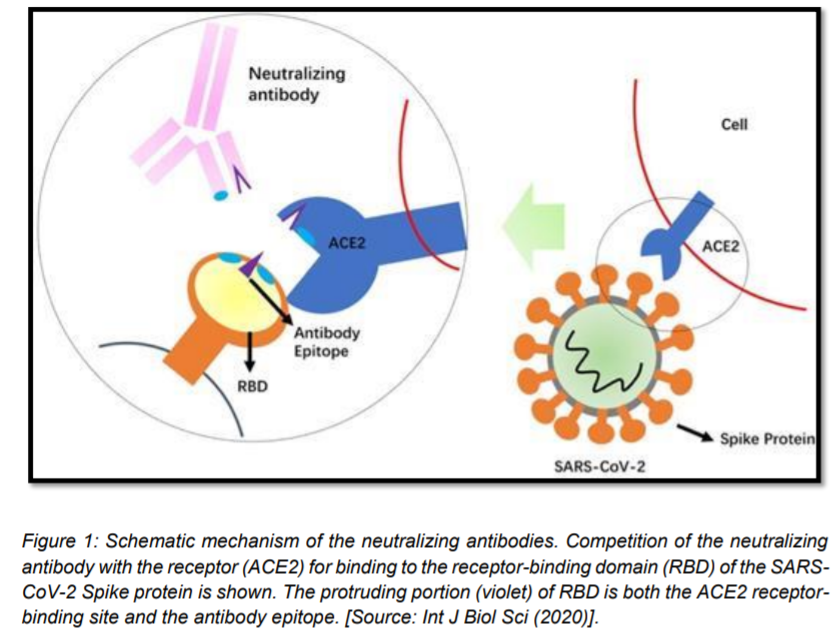
The current state of SARS-CoV-2 nAbs
Polyclonal antibodies isolated from COVID-19 recovered patients are currently in use to treat sick COVID-19 cases in the absence of any specific anti-SARS-CoV-2 monoclonal neutralizing antibody. However, many efforts have been going on toward isolating neutralizing mAbs that can be used either prophylactically or therapeutically post-exposure to prevent or treat COVID-19, respectively. Once isolated, the shortlisted nAb candidates will undergo rigorous in vitro testing for neutralizing and/or cross-neutralizing activity, efficacy assessment in COVID-19 animal models followed by preclinical and clinical trials before they are ready for human use [8].
Some of the notable SARS-CoV-2 nAbs that have been recently developed are:
- 47D11—first report of a human monoclonal antibody that cross-neutralizes both SARS-CoV-2 and SARS-CoV by binding a conserved epitope on the spike RBD [9]
- S309—neutralizes SARS-CoV-2 by engaging the S receptor-binding domain [10]
- B38 and H4—neutralizes SARS-CoV-2 by blocking the RBD of the viral spike protein from binding to the ACE2 receptor [11]
SARS-CoV-2 nAbs –from discovery to animal testing
Another recent research that adds to the current list of SARS-CoV-2 specific monoclonal antibodies came from Scripps Research Institute in collaboration with UC San Diego. Rogers et.al employed a deep mining approach coupled with high-throughput screening to isolate high-potency neutralizing antibodies against SARS-CoV-2 from the blood of recovered COVID-19 patients [6].[Figure 2]

They began by isolating SARS-CoV-2 specific memory B cells from donor plasma by capturing them with RBD and S antigen baits. The pool of SARS-CoV2 specific B cells expressing either anti-S or anti-RBD antibodies were flow-cytometrically sorted, and their heavy (H) and light (L) chain genes were PCR-amplified and cloned into expression vectors.
Transfection of the cloned H+L chain pairs into high-efficiency expression cell line yielded monoclonal IgGs in the supernatant. The secreted IgGs were tested for their efficacy to neutralize both live and pseudo SARS-CoV-2. [Pseudo SARS-CoV-2 (PSV)—a replication-deficient MLV (Murine Leukemia virus)-Luciferase vector possessing a truncated version of SARS-CoV-2 envelop protein (a truncation of S protein-∆18S allows pseudo virion maturation at the cell membrane because it removes the ER retention signal)]
In a virus neutralization assay, the authors first mixed the live SARS-CoV-2 or the pseudoSARS-CoV-2 with the IgG monoclonal antibodies. This premixing allowed the antibodies to bind to their corresponding antigenic sites, in this case, sites on S protein and the RBD of the virus. The virus/mAb premix was then applied to a monolayer of HeLa-ACE2 (target cells), to determine infection based on either plaque formation or luminescence (depending on whether the virus was live or pseudoyped). In this case, any antibody that blocks the viral interaction with ACE2 would not result in a plaque or luminescence, making it a neutralizing antibody as opposed to any other monoclonal antibody that binds to the S protein but does not block infection (also known as binding antibody).
As mentioned previously, not all viral protein binding antibodies are capable of neutralizing the virus, thus going by the same logic, the authors found that most of the potent neutralizers were RBD+ binders. Despite S+ binders comprising a large proportion of all anti-SARS-CoV-2 antibodies, they were not neutralizing types.
Next, the panel of potent neutralizers was characterized for their epitope specificities. The authors attached a biosensing platform with S and RBD antigens. They inundated the bound antigen surface with saturating concentrations of the neutralizing antibodies (found in the previous step) along with lower levels of other competing antibodies (4:1 ratio). This approach enriched only those nAbs that bound to non-competing sites. Predominant epitopes on RBD and S proteins were subdivided into—RBD-A, RBD-B, RBD-C and S-A, S-B, and S-C respectively.
Anti-RBD-A nAbs turned out to be the most potent neutralizers with IC50 values approaching single digits followed by an anti-RBD-B antibody while all non-RBD, but anti-S antibodies showed poor neutralization abilities. Since the extent of neutralization depends on the precise placement of the antibody at the interaction site of viral spike protein and host receptor, anti-RBD antibodies turned out to be the best candidates.
Two antibodies—anti-RBD-A nAb and an anti-S-B nAb were selected to evaluate their in vivo efficacy against SARS-CoV-2 in a Syrian hamster model. The anti-RBD-A nAb had an in vitro IC50 neutralization of 0.019 μg/ml against the pseudovirus, whereas anti-S-B nAb had IC50 neutralization of 22 μg/ml. Hamsters generally clear SARS-CoV-1 infection in one week so SARS-CoV-2 infection was followed for a week with weight loss and elevated lung viral titers as signs of infection. In a dose dependent manner, the antibody against RBD-A was able to reduce the disease burden while anti-S-B nAb failed to do so at any dose.
Conclusion and Outlook
Antibody-based therapy has come a long way from the administration of immune serum to passive infusion of highly selective human monoclonal antibodies. In the absence of any vaccine, monoclonal antibodies could be an alternative avenue to limit the impact of COVID-19. But this treatment modality is not without its fair share of pitfalls. Neutralizing antibodies in convalescent patients do not reach high titers, as exemplified by some recent studies, and moreover, the levels quickly subside following recovery. In this setting, the pipeline for the development of potent neutralizers derived from strong viral epitopes that can provide long-lasting immunity is both complicated and tedious given the time required for validation and screening of those antibodies for potency (in both animal models and clinical trials), and high costs of production before clinical use. Competition with other forms of treatment like small molecule antivirals, which are much more cost-effective to produce and distribute, further limits the scope of antibody treatments for viral diseases [2].
Nevertheless, passive immunotherapy with nAbs is quick and in the context of COVID-19, they can confer immediate protection to populations that are highly vulnerable to infection and places where outbreaks are rampant including nursing homes and meat-packing facilities. Additionally, passive infusion of nAbs in elderly individuals could provide rapid protection considering vaccine-elicited immune responses take time to mount in these individuals [2].
Neutralizing monoclonal antibodies to SARS-CoV-2 not only have the potential as prophylactic and therapeutic agents, but they can also inform new vaccine design through the identification of usable epitopes. Furthermore, broad cross-reactive nAbs that are capable of neutralizing other coronaviruses too can be the weapon to fight the current pandemic as well as any future outbreaks that humans might face.
- Pelegrin M, Naranjo-Gomez M, Piechaczyk M. Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents? Trends Microbiol. 2015;23(10):653-665. doi:10.1016/j.tim.2015.07.005
- Marovich M, Mascola JR, Cohen MS. Monoclonal Antibodies for Prevention and Treatment of COVID-19 [published online ahead of print, 2020 Jun 15]. JAMA. 2020;10.1001/jama.2020.10245. doi:10.1001/jama.2020.10245
- Walker LM, Burton DR. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat Rev Immunol. 2018;18(5):297-308. doi:10.1038/nri.2017.148
- Murin CD, Wilson IA, Ward AB. Antibody responses to viral infections: a structural perspective across three different enveloped viruses. Nat Microbiol. 2019;4(5):734-747. doi:10.1038/s41564-019-0392-y
- Burton DR, Williamson RA, Parren PW. Antibody and virus: binding and neutralization. Virology. 2000;270(1):1-3. doi:10.1006/viro.2000.0239
- Rogers TF, Zhao F, Huang D, et al. isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model [published online ahead of print, 2020 Jun 15]. Science. 2020;eabc7520. doi:10.1126/science.abc7520
- Giroglou T, Cinatl J Jr, Rabenau H, et al. Retroviral vectors pseudotyped with severe acute respiratory syndrome coronavirus S protein. J Virol. 2004;78(17):9007-9015. doi:10.1128/JVI.78.17.9007-9015.2004
- Jiang S, Hillyer C, Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses [published correction appears in Trends Immunol. 2020 Apr 24;:]. Trends Immunol. 2020;41(5):355-359. doi:10.1016/j.it.2020.03.007
- Wang C, Li W, Drabek D, et al. Publisher Correction: A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1):2511. Published 2020 May 14. doi:10.1038/s41467-020-16452-w
- Pinto D, Park YJ, Beltramello M, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody [published online ahead of print, 2020 May 18]. Nature. 2020;10.1038/s41586-020-2349-y. doi:10.1038/s41586-020-2349-y
- Wu Y, Wang F, Shen C, et al. A non-competing pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274-1278. doi:10.1126/science.abc2241