
Abbreviated Names:
Simukoko H., Mujuni, B.
* Address correspondence to:
Dr. Humphrey Simukoko, University of Zambia, School of Veterinary Medicine, Department of
Biomedical Sciences, P.O. Box 32379, Lusaka, Zambia. Postal code: 10101
Tel/Fax: +260-211-293727
Mobile: +260-975732243
Email: h.simukoko@unza.zm
Article type: Research Article
Received: July 11, 2020
Revised: July 20, 2020
Accepted: August 4, 2020
Citation: Simukoko H. and Mujuni, B.; Options for covid-19 therapeutics: aerosolized inhalation antibody conjugated nanoparticles, Biotechnology Kiosk, Vol 2, Issue 8, PP: 4-16 (2020); DOI: https://doi.org/10.37756/bk.20.2.8.1
Abstract
The world is currently faced with a very serious crisis to deal with the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2 or Covid-19) pandemic which started in Wuhan, China in December 2019 and has since spread throughout the world, wreaking havoc in many countries. Several efforts are being made to control the spread of the disease around the world and to find a cure or vaccine. As researchers frantically endeavor to identify remedies for covid19, there is the need to identify therapies that offer the quickest, safest actions and remedies that are relatively cheap. We propose the use of aerosolized inhalation antibody conjugated nanoparticles for the treatment of covid-19. It is hypothesized in this proposal that the conjugation of nanoparticles with antibodies and delivering the antibody-nanoparticle conjugate as an aerosol via the respiratory tract would provide the quickest and possibly more efficient and relatively cheap remedy against covid-19. The advantage of the inhalation route for delivering antibody conjugated nanoparticles is that since the medication is delivered directly to the affected site, higher doses will be delivered to the site with reduced systemic toxicity and reduced adverse effects on gaseous exchange. Our hypothesis is based on the current knowledge and observations in the areas of monoclonal antibody technology, advances in nanotechnology and Nano medicine as well as advances in inhalation therapeutics.
Key Words: Pandemic, Angiotensin-Converting Enzyme 2 (ACE2) receptor, Respiratory Epithelium, Virus, Nano medicine.
INTRODUCTION
The World Health Organisation (WHO) was informed of a cluster of cases of pneumonia of unknown cause detected in Wuhan City, Hubei Province of China on 31 December 2019. Later the causative agent of the pneumonia was identified by Chinese authorities to be the SARS-CoV-2 and the disease was named coronavirus disease 2019 (COVID-19) by WHO on 11 February 2020. Ever since its identification and characterization, the disease has been spreading world-wide causing high mortalities and morbidities. A group of experts Under the WHO’s coordination, with diverse backgrounds has been tasked to work towards the development of vaccines against COVID-19. Other scientists around the world are equally making frantic efforts to develop vaccines and therapeutics against COVID-19 [1].
Efficient and targeted delivery of the envisaged SARS-CoV-2 therapeutics to their sites of action will be cardinal in the treatment of covid-19. We therefore propose the use of aerosolized antibody-conjugated nanoparticles via direct inhalation into the respiratory system as one of the potential methods for the treatment of covid-19. Using aerosolized antibody-conjugated nanoparticles via direct inhalation has several advantages when compared to other potential remedies against covid-19. First, respiratory tract inhalation delivers medication directly to the site, thus enabling higher doses locally with less systemic toxicity. Second, inhaled drugs are likely to improve or at least have fewer adverse effects on gaseous exchange compared with other systemic routes of administration. On the other hand, systemically delivered drugs may be distributed widely thereby indiscriminately affecting other untargeted tissues which may result in unwarranted injury. Third, direct delivery of the drug to the lungs may permit reduction in the total medication dose and thus potentially lowering cost.
As the world grapples with the COVID-19 pandemic, it is prudent upon the scientific community to consider diligently all potential plausible solutions that may offer even a glimmer of hope now or in the future. Humankind is at the cross-roads for survival and therefore concerted efforts are extremely necessary to combat the COVID-19 pandemic.
Currently there is already an accumulation of knowledge on SARS-CoV-2 and other respiratory diseases. In addition, a number of intervention points for the possible treatment of Covid-19 have been suggested and some possible candidates are already being tested [2, 3]. Most of the potential therapeutic candidates such as hydroxylchloroquine, Ivermectin, remdesivir, interferon β1a and a few others are repurposed remedies that were/are used for the treatment of other conditions. These potential remedies currently being investigated for the possible treatment of SARS-CoV-2 are administered orally, intramuscularly (IM) or intravenously (IV) with a few exceptions such as interferon β1a (SNG001) that is administered via the respiratory route. Moreover, the modes of action of most of these adapted remedies are largely unknown.
Based on the current understanding of the structure of the SARS-CoV-2 including its cellular binding and cellular entry strategies, a number of plausible points for therapeutic interventions have been suggested. Cardinal amongst the therapies being suggested include camostat mesilate, a serine protease inhibitor [3, 4]. In addition, other small molecule drug compounds that might warrant further study have been identified that can limit viral recognition of host cells and/or disrupt host-virus interactions [5, 6]. Efficient and targeted delivery of the suggested SARS-CoV-2 therapeutics to their sites of action will be cardinal in the treatment of covid-19.
We therefore join the scientific community in proposing the use of aerosolized antibody-conjugated nanoparticles via direct inhalation into the respiratory system as one of the potential methods for the treatment of covid-19. The conjugation of nanoparticles to antibodies has been shown to improve efficacy in the treatment of certain diseases because the antibody-nanoparticle conjugate can provide new and enhanced properties as well as stability at the site of intended action [7, 8] The use of nanoparticles in medicine has gained a lot of research interest over the past few decades. Nanoparticles have been used to deliver drugs to specific types of cells including cancer cells [9, 10, 11]. Moreover, nanoparticles can be conjugated to antibodies that target specific epitopes on selected cells. The combination of nanoparticles with specific antibodies can ensure increased efficacy of the conjugate and optimal concentration of the payload at the site of action [12, 13, 14]
We hypothesize that the SARS-CoV 2 can be neutralized within the respiratory tract and its binding capacity to ACE2 receptors of the respiratory epithelium can be curtailed by the nanoparticle-antibody conjugates that would competitively bind to the ACE2 receptors. In this case, the antibody-nanoparticle conjugate will have dual action. On one hand, the nanoparticles will compete with the SARS-C0V-2 for binding to the ACE2 receptors while on the other hand the antibody moieties will bind and immobilize the free viral particles within the respiratory tract. Since the immobilized SARS-CoV-2 cannot effectively bind to the ACE2 receptors as a result of nanoparticle competitive binding and immobilization by the antibodies, the complex that is formed between the antibodies and the viral particles will eventually be eliminated by the mucociliary clearance system which normally clears particulate matter from the respiratory tract. Therefore, we further hypothesize that individuals that would receive the antibody-nanoparticle conjugate inhalation treatment would have a better outcome from a covid-19 infection than those who would not receive the treatment. The hypothesis would be tested by conducting clinical trials in which a group of covid-19 infected individuals would receive the therapy while another group would receive a placebo.
In this article, the hypothesis on the use of antibody-nanoparticle conjugate inhalation treatment is contextualized.
EVALUATION OF THE HYPOTHESIS
Inhalation of aerosolized medicines through the oral route is one of the most effective ways to get lifesaving medications to people with lung infections. The envisaged end product for our proposed covid-19 treatment is a hand-held covid-19 pressurized metered-dose aerosol inhaler or nebulizer. The user puts the mouth-piece of the inhaler in the mouth and presses down on the canister to release the medicine while breathing in to allow the medication to get into the respiratory tract (Figure 1). The hand-held inhaler is a small canister that works like a spray can.
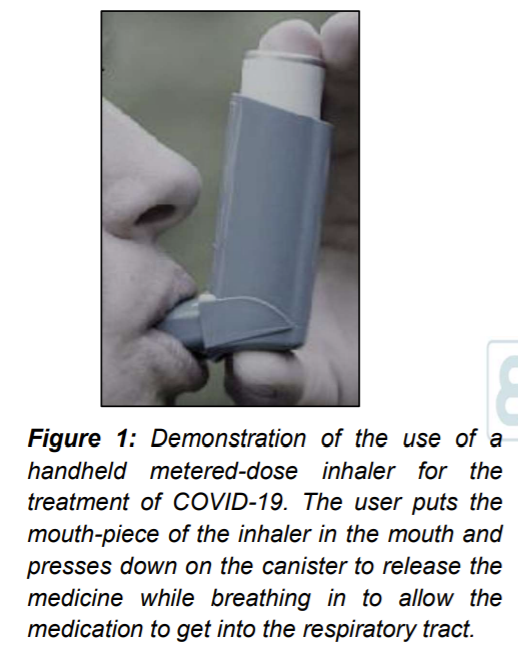
When a plunger on the inhaler is pushed down, the medicine is ejected in form of an aerosol which the individual breathes in (Figure 2).
The inhaler/nebulizer in our case would contain antibody-conjugated nanoparticles that target the ACE2 receptors on the respiratory cells and will at the same time bind the free SARS-CoV-2 spike proteins. The SARS-CoV-2 spike protein monoclonal antibody will be conjugated to the nanoparticle that has an epitope for binding to ACE2 receptors on respiratory epithelial cells (Figure 3).
When the medication containing the SARS-CoV-2 spike protein monoclonal antibody-nanoparticle conjugates is inhaled into the respiratory tract, the conjugates will competitively bind to the ACE2 receptors against the SARS-CoV-2 virus. In this case the conjugates would have a dual functionality. On one end, the free nanoparticle moiety would interact and bind to the ACE2 receptors while the free epitope on the monoclonal antibody would interact and bind any free SARS-CoV-2 viral particles. Thus, the viral particles would have insufficient or no ACE2 receptors to bind (Figure 4 and Figure 5).
The concept is based on the current knowledge of: (i) the SARS-CoV-2 structure (ii) SARS-CoV-2 interaction with respiratory cell receptors and cellular entry (iii) Nanoparticle technology or Nano medicine (iv) antibody-nanoparticle conjugation technology (v) pharmaceutical inhalation aerosol technology. In this article, we explain the basis of the hypothesis and how a covid-19 aerosolized inhalation antibody-nanoparticle conjugate therapy can be actualized based on the aforementioned body of knowledge.
How SARS-CoV-2 enters respiratory cells
Recent studies have shade more light on how the SARS-CoV-2 invades respiratory tract cells. SARS- CoV-2 gets into respiratory tract cells via the ACE2 receptor [15] The ACE2 receptor appears to be the passage for the virus into respiratory epithelial cells. The structural characteristics of the SARS-CoV-2 virus are similar to other coronaviruses. The virus contains four structural proteins known as the spike, envelope, membrane and nucleocapsid proteins [16].
The protein that interacts with the host cell receptors is chiefly the spike protein. In the initial step of receptor binding, the viral spike protein becomes cleaved into S1 and S2 by the host cell protease [15, 16, 17, 18, 19]. One of the host cell protease is the transmembrane protease serine 2 (TMPRSS2). The main function of the S1 subunit is to bind with the host cell surface receptors, while the function of the S2 subunit is to effect fusion of the virus with the host cell membrane [16, 17, 18, 20, 21].
Therefore, there are two potential therapeutic approaches against SARS-CoV-2. The first approach would be to either develop a vaccine that contains antigens derived from the spike protein, which can boost recognition of the virus by the immune cells or to develop monoclonal antibodies that bind to the coronavirus spike protein S1 subunit and block the interactions with the human cells ACE2 receptors. The second potential approach would be to target the transmembrane protease serine 2 which is responsible for entry and viral spread of the SARS-CoV-2. In our hypothesis, the aim is to produce monoclonal antibodies that bind the coronavirus spike protein.
Nanoparticles in medicine (Nanomedicine) and potential use in covid-19 treatment
Nanotechnology deals with matter generally in the 1-100 nm scale. The application of nanotechnology to medicine is known as nanomedicine. It involves the use of engineered materials of size 1-100nm length to develop novel therapeutic and diagnostic strategies [8]. Nanoparticles that are targeted to specific diseased cells or healthy cells of interest can reduce possible side effects. Moreover, nanoparticles can deliver a much higher payload to the intended site of action. If a drug is delivered in its conventional form, it is estimated that less than 1% of the drug actually makes it to the target site with 99% of the drug going to other parts of the body [9,10,11,12,13,14]. Nanotechnology is already used in many commercial medicinal products but many of them are not available to the consumer [14]. Given the urgent need for a Covid-19 remedy, nanotechnology offers grain of hope and may act as an enabling tool to skew promising medical therapeutics.
SARS-CoV-2 spike protein nanoparticles
Methods for generating SARS-CoV-2 full-length spike protein nanoparticles are available. The spike protein nanoparticles can be obtained and characterized by cleaving off the spike glycoproteins of the SARS-CoV-2 which reside on the surfaces of the virions. The purified full-length SARS-CoV-2 proteins have been determined to have molecular weight of approximately 160kDa by SDS-PAGE and approximately 25 nm in diameter [20, 21]. Moreover, recombinant spike protein nanoparticles that can bind to ACE2 receptors are available YP_009724390.1 [22]
In our hypothesized therapy, the spike protein nanoparticles will be conjugated to monoclonal antibodies against SARS-CoV-2.
Monoclonal SARS-CoV-2 antibodies
Monoclonal antibodies (Mabs) against coronaviruses or SARS-associated coronaviruses can be developed and characterized for reactivity to the SARS-CoV-2 spike protein (S), nucleocapsid protein (N), membrane protein (M), and envelop protein (E) using enzyme-linked immunoabsorbent assay (ELISA), radioimmunoprecipitation, immunofluorescence, Western Blot and microneutralization assays (32). Indeed, single stranded DNA (ssDNA) aptamers with high binding affinity to the SARS-CoV proteins can be identified from DNA libraries [22, 23, 24. 25.]. In our hypothesis, the aptamers that bind to the nucloecapsid protein will be used.
Antibody-nanoparticle conjugation
Antibody-conjugated nanoparticles offer great opportunities to overcome limitations found in conventional therapeutics [14]. They combine the advantages given by the nanoparticles with the ability to bind to their target with high affinity and improve cell penetration given by the antibodies [8,9,10]. Antibody-conjugated nanoparticles could play a critical role in medical therapeutics [14]. In biotechnology, antibodies could be used to carry several elements such as drugs and nanoparticles in diagnostic procedures or even in therapy to bind to specific targets. The conjugation of antibodies to nanoparticles can generate products that combine the properties of the antibody and nanoparticle whereby the hybrid product would show adaptability and specificity. By conjugating different moieties to the nanoparticles, their application can be widened in different fields including therapies for covid-19 and can provide them with novel or boosted properties [7,8].
Aerosolized inhalation antibody-conjugated nanoparticles and potential in covid-19 treatment
In many instances, respiratory diseases are treated using inhalation therapy. Currently there is significant research in pulmonary drug delivery using solid colloidal nanoparticles in the treatment of many respiratory diseases [24].
The inhalation drug administration route is often used for the management of respiratory diseases. Compared with other routes of administration, inhalation offers a number of advantages in the treatment of these diseases. For example, via inhalation, a drug is directly delivered to the target organ, delivering high drug concentrations but low systemic drug concentrations. Therefore, drug inhalation is typically associated with high pulmonary efficacy and minimal systemic side effects [24, 25. 26, 27, 28, 29] and would thus be useful in covid-19 therapy.
HYPOTHESIS TESTING
In order to evaluate the effectiveness and safety of the medication, clinical trials will be performed by monitoring the effects on groups of people. The four phases of the classical clinical trial will be followed.
Phase I trial will be done to assess safety and side effects as well as to determine the correct drug dosage. The drug will be administered to 20 but less than 100 healthy individuals.
Phase II trial. In this trial phase, about 100 to 300 volunteers will be given the drug in order to assess the effectiveness of the durg. In this phase the aim will be to obtain preliminary data on whether the drug works in people who have COVID-19. In addition, this phase will continue to assess the safety, including short-term side effects.
Phase III trial. In this phase, more people volunteers (500-1000) will be involved in order to gather more information about safety and effectiveness and to study different populations and different dosages as well as in combination with other drugs.
Phase IV trial. If the drug is approved by the relevant regulatory agencies, the drug’s effectiveness and safety will be monitored in large, diverse populations.
Potential Advantages and Disadvantages of Aerosolized Inhalation SARS-CoV-2 therapy
Inhalation route has many important advantages compared to other routes of drug administration and should be seriously considered in potential SARS-CoV-2 therapies. First, respiratory tract inhalation delivers medication directly to the site, thus enabling higher doses locally with less systemic toxicity. Second, inhaled drugs are likely to improve or at least have fewer adverse effects on gaseous exchange compared with other systemic routes of administration. On the other hand, systemically delivered drugs may be distributed widely thereby indiscriminately affecting other untargeted tissues which may result in unwarranted injury. Third, direct delivery of the drug to the lungs may permit reduction in the total medication dose and thus potentially lowering cost.
The inhaled route may also have some disadvantages. Inhaled aerosolized SARS-CoV-2 therapies may not be tolerated by some individuals due to sensitization or direct irritation on the respiratory airways by the drug or the excipients. In addition, the dosage may not be very precise because of variances in breathing patterns and the challenges in determining exactly how much medication reaches the targeted regions of the lungs. Also, the cumbersomeness and difficulty in effective operation of inhalation devises by the user may cause erroneous dose administration [26, 27, 29].
Potential cytotoxicity of the proposed treatment and possible remedial actions
The main active ingredients of the proposed treatment will be the antibodies conjugated with the SARS-CoV-2 recombinant spike protein nanoparticles. The active ingredients will be contained in an inert non-toxic excipient. The recombinant SARS-CoV-2 spike protein nanoparticles that will be used in the proposed treatment have been assessed by others for safety and binding affinity to ACE2 receptors [22]. Therefore, no major toxicity is expected from the nanoparticles. Also the antibodies (aptamers) that will be used in the proposed treatment will be ssDNA whose affinity and safety are already known. The aptamers will be identified from DNA libraries [22,23,24,25]. Thus, the active ingredients in the proposed treatment will be compatible to the tissues within the respiratory tract. Qualified medical personnel will be on hand in cases of inadvertent adverse reactions to either the excipient or any of the active ingredients and appropriate medical interventions will be immediately implemented.
CONCLUSION
The advantages of using inhaled aerosolized antibody-conjugated SARS-CoV-2 therapies would seem to outweigh other routes of administration. It is therefore the contention of the author that potential remedies for the treatment of SARS-CoV-2 should be administered preferably through the respiratory tract route as inhalation aerosols conjugated to nanoparticles. Respiratory route administration of potential SARS-CoV-2 therapies would be advantageous compared to other routes such as the oral route, IM or IV routes. The respiratory tract route would provide a quick and relatively safe route for SARS-CoV-2 therapies preferably using aerosolized antibody-conjugated nanoparticles.
However, researchers in SARS-CoV-2 therapeutics before considering clinical trials for aerosolized antibody-nanoparticle conjugates should ensure the following: (i) Identification of appropriate nanoparticles for conjugation with SARS-Cov-2 spike antibodies [30, 31, 32, 33, 34, 35] (ii) Production of monoclonal antibodies that are specific to the SARS-CoV-2 spike proteins (iii) Conjugation of monoclonal antibodies with nanoparticles and , (iv) Production and optimization of aerosolized antibody-nanoparticle conjugates using easy to use inhalation delivery systems.
ACKNOWLEDGEMENTS
The authors are grateful to the University of Zambia for providing unlimited internet access and library facilities.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest to disclose
REFERENCES
1. WHO. Emergencies preparedness, responses: Pneumonia of unknown cause, diseases outbreak news. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/. 2020. Accessed on 08/05/2020.
2. Guo Y, Cao Q, Hong Z, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020; 7:11, DOI: https://doi.org/10.1186/s40779-020-00240-0.
3. Mahase E. Covid-19: What treatments are being investigated? BMJ. 2020; 368: DOI:10.1136/bmj.m1252 m1252.
4. Liu C, Zhou Q, Li Y, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020; 6, 3: 315-31, DOI: https://doi.org/10.1021/acscentsci.0c00272.
5. Smith M & Smith CJ. Repurposing Therapeutics for COVID-19: Supercomputer-Based Docking to the SARS-CoV-2 Viral Spike Protein and Viral Spike Protein-Human ACE2 Interface. ChemRXIV. 2020, DOI: 10.26434/chemrxiv.11871402.v3.
6. Kruse RL. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020. 9:72, DOI: 10.12688/f1000research.22211.2.
7. Mullen DG, Fang M., Desai A, Baker JR, Orr BG, Banaszak HM M. A quantitative assessment of nanoparticle-ligand distributions: implications for targeted drug and imaging delivery in dendrimer conjugates. ACS nano. 2010; 4(2): 657–70, DOI: https://doi.org/10.1021/nn900999c.
8. Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008; 83(5):761-69, DOI: 10.1038/sj.clpt.6100400.
9. Ma X, Xiong Y, Lee, LTO. Application of Nanoparticles for Targeting G Protein-Coupled Receptors. Int. J. Mol. Sci. 2018; 19(7): 2006, DOI: 10.3390/ijms19072006.
10. Yameen B, Choi WI, Vilos C, Swami A, Shi JJ, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. 2014; 190: 485–99, DOI: 10.1016/j.jconrel.2014.06.038.
11. Jain R.K & Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010; 7: 653–64, DOI: https://doi.org/10.1038/nrclinonc.2010.139.
12. Richard S, Boucher M, Saric A, et al. Optimization of pegylated iron oxide nanoplatforms for antibody coupling and bio-targeting. J. Mater. Chem. B. 2017; 5: 2896–907, DOI: https://doi.org/10.1039/C6TB03080G.
13. Qinqin H, Hua L, Lihua W, Hongzhou G, Chunhai F. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem Rev. 2019; 119 (10): 6459-506, DOI: https://doi.org/10.1021/acs.chemrev.7b00663.
14. Murthy SK. Nanoparticles in modern medicine: state of the art and future challenges. Int J Nanomedicine. 2007; 2(2): 129–41, DOI:
15. Gallagher TM & Buchmeier MJ. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001; 279(2): 371–4, DOI: 10.1006/viro.2000.0757.
16. Belouzard S, Millet JK, Licitra BN. Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4 (6): 1011-1033, DOI: 10.3390/v4061011.
17. Heald-Sargent T & Gallagher T. Ready, Set, Fuse! The Coronavirus Spike Protein and Acquisition of Fusion Competence. Viruses. 2012. 4(4): 557–580, DOI: 10.3390/v4040557.
18. Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: Cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013; 100(3): 605–14, DOI: 10.1016/j.antiviral.2013.09.028.
19. Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. U.S.A. 2009; 106(14): 5871–6, DOI: https://doi.org/10.1073/pnas.0809524106.
20. Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor [published online ahead of print, 2020 Mar 30], Nature 581, 215–220 (2020), DOI: https://doi.org/10.1038/s41586-020-2180-5.
21. Luan J, Lu Y, Jin X & Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochemical and biophysical research communications. 2020. 526(1), 165–169, DOI: https://doi.org/10.1016/j.bbrc.2020.03.047.
22. Groff K, Brown J, Clippinger AJ. Modern affinity reagents: Recombinant antibodies and aptamers. Biotechnol Adv. 2015;33(8):1787‐1798, DOI: https://doi.org/10.1016/j.biotechadv.2015.10.004.
23. Xu Y, Lou Z, Liu Y, et al. Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J Biol Chem. 2004. 279(47):49414‐49419, DOI: 10.1074/jbc.M408782200.
24. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020. 367(6483):1260‐1263, DOI: 10.1126/science.abb2507.
25. Cho SJ, Woo HM, Kim K-S, Oh J-W, Jeong YJ. Novel system for detecting SARS coronavirus nucleocapsid protein using an ssDNA aptamer. J. Biosci. Bioeng. 2011. 112:e1347-e4421, DOI: https://doi.org/10.1016/j.jbiosc.2011.08.014.
26. Aysu Y, Mesut A, Mine O. Nanopharmaceuticals: Application in inhaler systems. In: Emerging Nanotechnologies in Immunology. The Design, Applications and Toxicology of Nanopharmaceuticals and Nanovaccines Micro and Nano Technologies. 1st Edition. (Ranjita Shegokar and Eliana B. Souto, eds.). 2018. pp: 165-201.
27. Labiris NR. & Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003; 56(6): 600–12, DOI: 10.1046/j.1365-2125.2003.01893.x.
28. Borghardt MJ, Kloft C, Sharma A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can Respir J. 2018, DOI:10.1155/2018/2732017.
29. Hill NS, Preston IR, Roberts KE. Inhaled Therapies for Pulmonary Hypertension. Resp Care. 2015. 60 (6): 794-805, DOI: https://doi.org/10.4187/respcare.03927.
30. Coleman CM, Liu YV, Mu H, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014. 32(26), 3169–3174, DOI: 10.1016/j.vaccine.2014.04.016.
31. Liu YV. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011. 29(38): 6606–6613, DOI: 10.1016/j.vaccine.2011.06.111.
32. Li J. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013. 26(2):126–132, DOI: 10.1089/vim.2012.0076.
33. He Y. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004. 324(2):773–781, DOI: https://doi.org/10.1016/j.bbrc.2004.09.106.
34. Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009. 7(3):226‐236, DOI: https://doi.org/10.1038/nrmicro2090.
35. Tripp RA, Haynes LM, Moore D et al. Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins J. Virol. Methods, 128 (2005), pp. 21-28, DOI: 10.1016/j.jviromet.2005.03.021.