Folate receptor-mediated Therapeutics and Imaging
Lokesh Chandra Mishra1, Jyotsna Gupta2, Satyam Ravi Dwivedi3 and Gauri Mishra4*
1Department of Zoology, Hansraj College, University of Delhi, Delhi-110007, India
2Department of Experimental Medicine and Biotechnology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh-160012, India
3Department of Zoology, Bhaskaracharya College of Applied Sciences, University of Delhi, Delhi-110075, India
4Department of Zoology, Swami Shraddhanand College, University of Delhi, Delhi-110036, India
Abstract
Cancer is the second leading cause of death globally. Before a probable treatment approach is adopted based on early-stage diagnosis, there is a requirement for specific constructive and efficacious strategies. Identifying solid tumors through targeted detection of cancerous cells is one of the most reliable approaches to treatment. Over the past decade, the investigation of folate receptors (FR) has led to the development of several novel cancer treatments. Many tumor types exhibit high overexpression of FR, which was found to be associated with tumor progression and prognosis. Monitoring FR expression levels can prove a useful criterion as it is correlated with the advancement and prognosis of tumors. Due to the strong affinity of the FR towards folate and folate-conjugates, it can be designated as a tumor-associated antigen that is involved in the active transportation of the bound cargo within cells through an endocytic process. A wide range of payloads, from tiny radioactive imaging agents up to massive DNA-containing formulations, may be delivered to FR-positive cells using folate or an analog of it as the ligand. The FR targeting anticancer therapies are being developed currently and the response-predictive aspect of FR expression could serve as a biomarker for these treatments. By boosting delivery to the target tissue as well as an improved target-non-target tissue ratio, targeted drug delivery systems aim to increase the therapeutic windows of molecules. In turn, this results in a decreased minimum effective dose of the drug, lower drug toxicity, and improved treatment efficacy at comparable plasma concentrations. Targeted delivery is especially appealing for drugs with small therapeutic windows and/or those activated at very low concentrations due to the few targeted receptor sites on the specified target tissue. This review discusses recent developments in FR-mediated targeting for therapeutics and its applications in imaging, as well as highlights their potential and anticipated challenges.
Keywords: cancer, folate receptor, folic acid, anticancer therapy, tumor marker, endocytosis
*Corresponding Author: Dr. Gauri Mishra
E-mail address: gaurishukla1@gmail.com
DOI: https://doi.org/10.37756/bk/23.5.7.1
Article type: Review
Received: April 8, 2023
Revised: June 1, 2023
Accepted: June 2, 2023
Please cite this article as: Mishra LC et. al., Folate receptor-mediated Therapeutics and Imaging. Biotechnol. kiosk, Vol 5, Issue 7, PP: 1-13 (2023); DOI: https://doi.org/10.37756/bk/23.5.7.1
Introduction
Receptor-targeted drug delivery is affected by the constitution of the targeting ligand and the properties of the accompanying cargo and may offer the following two benefits over standard non-targeted therapies: Both receptor-mediated targeting and receptor-targeted delivery have the potential to lessen collateral toxicity to normal tissues by (1) increasing the total absorption of the desired drug by the treated cells, and (2) reducing drug deposition in non-pathologic cells. Because it can help accomplish these benefits, there is a spike in folate receptor (FR) use for various targeted drug-delivery applications. While it is yet to be confirmed whether FR-targeting significantly enhances the net uptake of low molecular weight chemotherapeutic compounds, except for some antifolates, it has been shown to increase intracellular delivery of attached biomolecular parcels. These parcels include proteins, liposomes, viruses, gene therapy vectors, antisense oligonucleotides, imaging compounds, polymeric drug carriers, and neutron activation agents. (1-11)
There might be possibilities that these targeting ligands match the effectiveness of folate in efficiently delivering a few of the drugs discussed above, but Folate Receptor controlled endocytosis still enjoys an edge that is difficult for rival antibody- and ligand-targeted technologies to match. As a result, cells often internalize antibodies, hormones, and other relevant ligands to remove them from the surface of the cells which means that as soon as a hormone has undergone signaling through its receptor, it must be relieved and managed in a way that it gets destroyed so to prevent uninterrupted signaling for ensuring the stability and life of the cell. Therefore, the majority of targeted ligands are absorbed and directed toward the lysosomes for apoptosis. As a consequence, the major cargo associated with folic acid is not transported to lysosomes, but rather discharged into the cytosol or stored in endocytic regions because it is required for critical cell functions. (12, 13) However, in none of these instances is a sizable portion digested or eliminated. In some cases, the linked cargo associated with the folate seems to be directly transported to the nucleus. This property is crucial for delivering macromolecular or hydrolytically sensitive medications as they are prone to inactivation due to digestion by various hydrolytic enzymes localized in the lysosomes. (14)
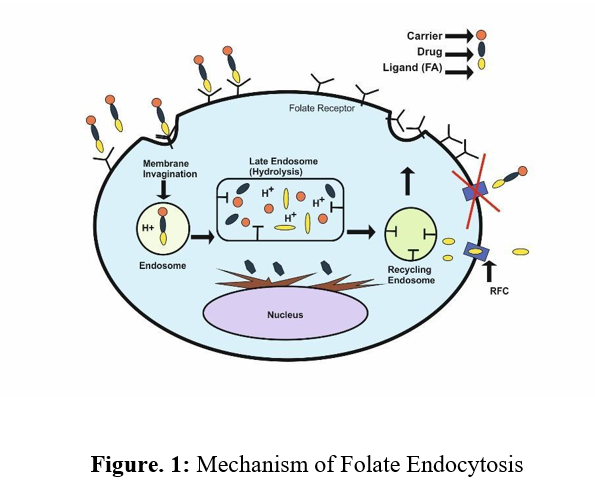
Folic acid and its reduced byproducts are essential for eukaryotic cells in one-carbon transfer processes that contribute to the production of nucleotide bases. It is not surprising that cellular absorption of the vitamin is crucial for cell survival and growth. The absorption of folates by cells is facilitated by a low-affinity reduced folate carrier (Km 1 mM), which is present in almost all body cells, and a high-affinity glycosylphosphatidylinositol-linked Folate Receptor (FR) (KD 100 pM), which is more limited in distribution. (15) The folate receptor is involved in the effective transportation of both folic acid and various kinds of cargo associated with folate. However, the reduced folate carrier helps only certain reduced forms of folic acid to travel (i.e., chemotherapeutics, liposomes, imaging agents, proteins, nanoparticles, etc.). When conjugated folates adhere to an FR on a cell surface, Figure 1 shows how the FR present on the surface of the cell after binding to the conjugate aids in the release of an initial quantity of the therapeutic load into the cytoplasm which is referred to as the final stage in the receptor-mediated absorption of folate drug ligand-conjugates. (16) As a result, some (but not all) folate conjugates are released from their receptors when the compartments of the endosome are acidified to a pH of 5 after the formation of an endocytic vesicle following the invagination of the membrane and internalization. (17).
The separation of the membrane-bound FR from released conjugate/free medicines is subsequently made possible by the transportation of the acidic components of the endosomal compartments for recycling. The endosome is observed to be breached by released folate conjugates by an unidentified mechanism, leading to drug deposition in the cytoplasm. The transport of more folate-linked medications into the cell is made possible via membrane-bound FR, which is mostly recycled and comes back to the cell surface. The recycling proportion of cancer cells fluctuates in real-time, from one cycle every 4 hours to one cycle every 12 hours. The recycling rate may be higher in other FR-positive cells. The introduction of hydrolytically sensitive items like genes and ribozymes into cells is made possible because only a few of the folate conjugates enter lysosomes for degradation (18). As mentioned previously, FR expression is minimal in healthy cells but is frequently abundant in cancer cells. In epithelial malignancies of the brain, nose, throat, mammary gland, lung, ovary, colon, and prostate, for instance, overexpression of FRs is observed (19, 20). In instances of acute and chronic myelogenous leukemia i.e. hematological cancers of myeloid origin, FRs are also overexpressed (21, 22). It has been found that FR expression and a tumor’s grade and histological stage are strongly correlated. FR expression is typically significantly higher in highly undifferentiated metastatic tumors compared to localized, low-grade malignancies.
Function of Folate
One-carbon metabolism: The body’s sole apparent use for folate coenzymes is to facilitate the transfer of one-carbon molecules. Folate coenzymes participate in several processes essential to the metabolism of nucleic acids and amino acids as donors and acceptors of one-carbon units.
Nucleic acid metabolism: Through two distinct routes, folic acid coenzymes are essential for DNA metabolism.
Folate coenzymes are required for the synthesis of DNA through its precursors.
The biosynthesis of methionine requires a folate cofactor, and the production of S-adenosylmethionine depends on the formation of methionine (SAM). One carbon unit methyl group donor, SAM, is utilized in a broad range of methylation activities at the biological level, which includes the methylation of particular locations in several DNA and RNA. DNA methylation can be a crucial target for preventing cancer as it plays an important role in the same.
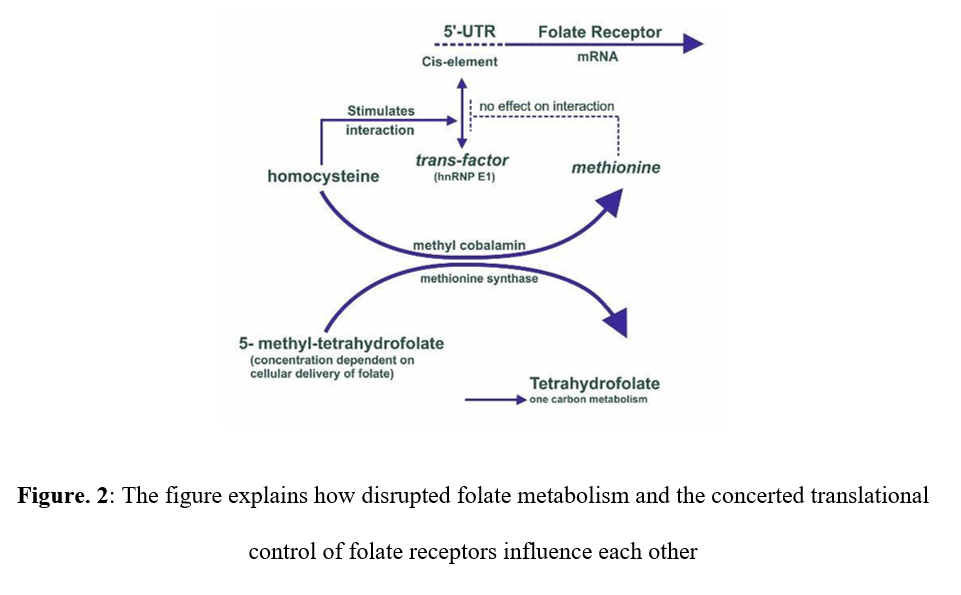
Amino acid metabolism: The body requires the assimilation of several significant amino acids which is achieved by successful metabolism in the cells, which calls for the use of folate coenzymes. A folate coenzyme and a vitamin B12-dependent enzyme are the two essential requirements for the production of methionine from homocysteine. The thymidylate synthase reaction step is responsible for the detrimental effects of folate deprivation that are evident in the fast-increasing red blood cell metabolism. Henceforth, there is a direct correlation between the disturbed levels of folate metabolism and the control of folate receptor expression (Figure 2). As in cancer chemotherapy, when there is a need to inhibit rapidly growing cells, dihydrofolate reductase (DHFR) is inhibited.
Folate Metabolism
One-carbon molecules need to be transported to various metabolic destinations, and folic acid acts as a carrier. A variety of oxidation states exists for this one-carbon component, and from a clinical perspective, methyl-tetrahydrofolate (methyl-THF) and methylene-THF are the two most significant forms of folic acid. The relevance of these chemical forms and the sequence of the metabolic pathway experienced by these intermediates are depicted below. The various reactions are then interconnected to highlight the main one-carbon transfer processes. The first step in the folate metabolism pathway involves the transfer of the methyl group from serine. The synthesis of 5, 10-methylene-THF from THF is mediated by the transfer of the methyl group from serine to glycine (Figure 3). DHF and THF obtained from natural sources are then polyglutamated and are required to be enzymatically transformed to mono glutamate to be absorbed from the small intestine. In another approach, 5, 10-methylene-THF can be transformed into 5, 10-methenyl-THF, and finally, 10-formyl-THF.
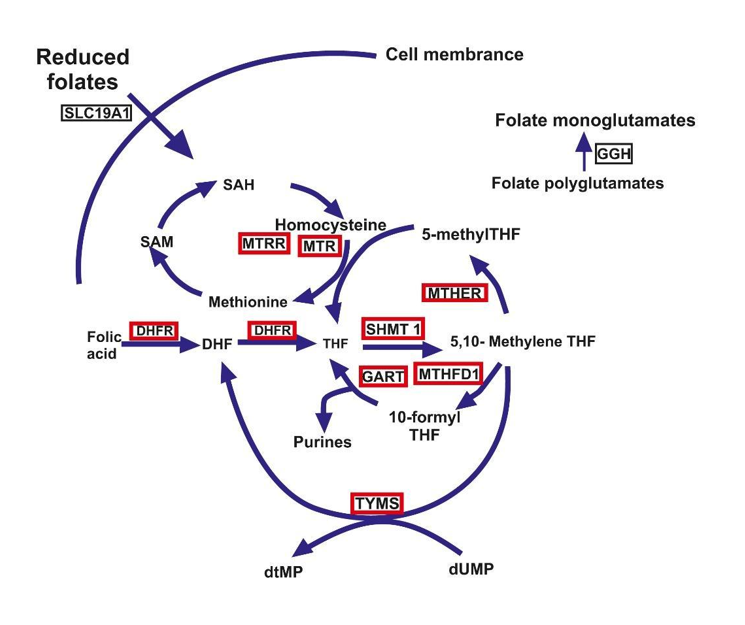
Figure. 3: Folate Metabolism
For the reasons listed, vitamin-based drug delivery systems offer significant benefits over other drug delivery methods, such as monoclonal antibodies:
The effective delivery of the conjugate into the tumor is possible only due to the Folate’s relatively lower size, (molecular weight is 441) whereas typical monoclonal molecular weights exceeding 150,000 makes it simpler to reach cancer cells.
The possibility that the folate drug combination will attach to the receptor is increased since the folate affinity for its receptor on cancer cells is 100x higher than when the affinity for monoclonal antibodies is compared.
A significant quantity (20–60 million) of folate drug conjugates can be introduced into the cell in 4 hours because the folate receptor on cancer cells can be easily recycled.
The lysosomes inside the cell do not break down folate conjugates. Cells possess compartments called lysosomes that break down molecules to eliminate potentially hazardous compounds. Lysosomes do not attack folate conjugates because folate is transported into the cell to be consumed rather than destroyed.
Folate is a natural chemical that does not cause the body to launch an immune reaction, which is crucial in the availability of various types of medications i.e. drug delivery, consisting of several genes and proteins. Consequently, it may be given more than once. However, unless they are humanized (a costly procedure), monoclonals cause a probable response of the immune system that prevents the immature meetup of antibodies with the cancer cell.
Last but not least, folate is easily obtainable, simple to make, stable during synthesis and storage, and is known to be a crucial constituent of a large number of medications.
Folate Receptor as a Tumor Marker
The folic acid (FA) and folic acid receptor (FR) appear to have a 1:1 stoichiometry and the latter possesses a single-chain glycoprotein structure on the cell surface. This structure mediates a relatively high visible affinity binding and the absorption of the vitamin by receptor-mediated endocytosis. The primarily circulating folate coenzyme, (6S)-5-methyltetrahydrofolate, and several antifolate medications all bind to the folate receptor. The Folate receptor has been identified, described, and cloned in three different isoforms.
Several isoforms of FR have been identified from
- i) Human (i.e. hFR-α, hFR-β, and hFR-γ) and
- ii) Murine (i.e. mFR-α and mFR-β) sources
A variety of Folate receptor structures consists of 16 cysteine residues conserved sequences that share approximately 70% sequence identity and are termed isoforms. These FR isoforms are all associated with prominent functions. With Kd values of 10-10 mol/L and 10-9 mol/L, respectively, FR-α and FR-β demonstrate remarkably comparable affinities for FA despite the difference in their carboxy-terminal sequences. However, the stereospecificities of FR-α and FR-β for reduced folate coenzymes are different, with FR-α having a 50-fold greater affinity than FR-β for the physiological (6S) diastereoisomer of N5-methyltetrahydrofolate (15, 21, 23). Importantly, even the FR’s α-isoform binds FA ten times more strongly than any of the vitamin’s more reduced forms do.
There is an uneven distribution of FR isoforms throughout the body’s numerous tissues and cell types. Instead, FR-α is mostly expressed on healthy epithelial cells and is overexpressed in cancerous tissues made of the same cell types.
The Folic acid receptor is considered a preferred target for the delivery of tumor-specific drugs because:
(1) The levels of the Folic Acid receptors are elevated in a variety of human cancers, such as brain, breast, myeloid cell, lung, kidney, and ovarian.
(2) The folate receptor is located on the membrane facing the external apical face of the polarized epithelia, which can severely restrict access to it in normal tissues that express it.
(3) The density of the Folate receptor is contemplated to be an efficient biomarker since its level increases along with the cancer progression.
Folate-linked therapies can therefore be used to target malignancies that are hardest to cure using traditional means. Folic acid is found to be coupled with both medications of low molecular weight and macromolecular complexes as a means of directing the molecular conjugates to cancerous cells to take advantage of these characteristics of folate receptor expression. In vitro studies have revealed that adding folic acid to macromolecules improves their delivery to cancer cells that express the aforementioned folate receptors.
FR as a diagnostic and therapeutic target
A plethora of advantages can be enlisted for using the FR as a diagnostic and therapeutic target. (24, 25) In the majority of instances of proliferating non-tumor tissues, FRα is found to be not available or inaccessible to circulatory pathways as it is located on the luminal surface of epithelial cells. In contrast, in the cases of malignant tissues, FRα is accessible through circulation as it is expressed all over the cell. FR carries the potential capability of binding to a molecule of small and comparatively innocuous nature, i.e., folic acid that forms chemical conjugation with other molecules and is known to significantly infiltrate the developed solid tumors. Once FR is bound to a folate conjugate, it gets internalized into the cell, and the FRα is recycled rapidly to the surface of the cell via the FR-mediated endocytic pathway. (25, 26)
Folate-conjugated nanoparticles in the detection and treatment of tumors
The principal application of FR in recent nanotechnology breakthroughs is the development of imaging techniques for detecting tumor localization and therapeutic drugs. Folate-conjugated IO characteristics for the treatment of intracellularly localized hyperthermia of solid tumors that are abundant in FR are being investigated. (28) Other recent methods that have been tested in vitro and make use of nanoparticle technology include the use of iron oxide nanoparticles (27), fluorescent silica nanoparticles conjugated with folate (29), hydrophobic nanocrystals (30), destruction of cancerous cells by near-infrared agents that are transported via folate-containing carbon nanotubes (31) and lipoprotein-based nanoplatforms that alters the de novo route taken up by the lipoproteins from their normal receptor to the cancer-associated FR i.e, reroutes it. (32)
Customized cancer therapies utilize the role of FR expression
Certain tumor subtypes express FR, which may be useful as a biomarker for a therapeutic approach that is FR-directed as a component of a tailored strategy toward effective treatment. The creation of reliable, relevant, and usable measuring assays is necessary for the assessment and confirmation of tumor biomarkers. (33) Numerous semiquantitative and quantitative techniques are required for the evaluation of FRα levels in tumor biopsy tissue samples. But many such methods have inherent difficulties, especially in a therapeutic situation. (34-41) The in vivo use of an imaging agent that acts as a companion, which enables the noninvasive, whole-body, real-time evaluation of FRα expression, is an appealing alternative for making it easier to monitor FR status throughout the treatment without the involvement of invasive procedures for obtaining tissue biopsies. The expression of FR in tumors can vary because the molecular features of malignancies might alter over time (42-44). The link between folic acid and FR binding provides a valuable carrier that enables probes involved in imaging to be targeted to FR-expressing cells. The probe exhibits high-affinity binding towards the FR upon conjugation with a folic acid molecule, which results in the emission of an imaging signal. Magnetic resonance imaging, fluorescence imaging, single-photon emission computed tomography (SPECT), computed tomography, positron emission tomography (PET), and ultrasound imaging is the currently available techniques that may identify probe/folic acid conjugates. Etarfolatide (EC20), one of the several folate conjugates that have been examined from the perspective of tumor imaging, is of special interest. (45-52) A short and linear folate-linked peptide and 99mTechnetium (Tc) make up the imaging agent complex etarfolatide. (53) A typical radiographic tracer is 99mTc. After being introduced into the bloodstream, the folic acid complex localizes to tissues that express FR and attaches to the FR with a high affinity. 99mTc-etarfolatide is highly absorbed by FR-positive tumors (17% of the injected dose per gram of tissue) and rapidly accumulates within the target tissue. (45) To see the expression of FR, utilize SPECT. (54) The kidneys filter out extra probe conjugates. This lessens the general background and makes it possible to acquire the photographs rapidly. (45)
Targeting FRα Imaging and theranostics
The field of cancer imaging is exploring FRα and its tumor specificity. Many alternate approaches have been developed and this has efficiently reinforced the imaging of FRα-positive tumors for this FRα-targeted contrast-enhanced MRI has been explored widely. For example, folic acid is attached to a polyamidoamine dendrimer to develop a folate-conjugated dendrimer polychelate. As a result, there is an improvement in contrast enhancement relative to non-targeted contrast agents. This causes targeted tracers to accumulate in FR-expressing tumors. (55) A folic-acid-derived molecule combined with a carboxylate-containing oxide of iron exhibited high retention in FR-positive tumor cells in a study involving breast cancer cell lines and xenograft models. (56) Combining heparin-folic acid micelles and superparamagnetic iron oxide nanoparticles produced identical results. (57) Radiolabeled folate derivatives are also currently being researched. 111In-diethylenetriaminepentaacetic acid-folate was delivered in phase I/II trial to get a full-body single-photon CT emission image of women diagnosed with endometrial cancer or ovarian cancer. When combined with other radiography techniques, the radiotracer concentration present in all probable malignantly developed lesions demonstrated good levels of sensitivity for the identification of malignancy (50). In xenograft models of mice with ovarian cancer exposed to radiolabeled mirvetuximab soravtansine (an FR that targets ADC), an antibody-based companion diagnostic molecule, 89Zr-DFO-M9346A, is being employed. Up to this point, this drug has shown a distinct level of encouraging tumor-to-background resolution in this animal. (58) The intraoperative imaging of tumors in mouse models has been possible for more than ten years thanks to non-radiolabeled techniques like fluorescent probes connected to folate (59). These methods must be optimized appropriately to help surgeons do better resections and enable visible improvements in patients with FR-expressing tumors, such as those with lung, ovarian, and breast malignancies (60–62).
Conclusions
The FR is involved in the development and progression of cancer to a great degree. As a result, therapies that target FR require dependable methods for detecting tumors that are positive for FR to assist in identifying individuals who are candidates for treatment. The folate receptor is emerging as a potentially effective diagnostic and therapeutic target in cancer treatment. In many cancerous cells, folate receptors found on the cell surfaces are overexpressed to a high degree. The overexpression can be exploited by therapeutic agents to target malignant tissues through various pathways directly. Even if treatments that make use of FR have shown to be effective, it is still necessary to conduct additional research and testing on such treatments in human beings. One of the most important things that need to be done is to research the right amount of dosage and the possible long-term consequences of using nanoparticle-based drug delivery as the treatment approach for cancer. For patients who take folic acid supplements, there is a need for additional research into the interactions that can occur between the effects of folate and antifolate. Countries that already fortify their meals with folic acid should also consider this recommendation.
The successful utilization of folate conjugates highlights the promising potential of these receptors for cancer management.
References:
- Leamon, C.P.; Low, P.S. Selective targeting of malignant cells with cytotoxin–folate conjugates. J. Drug. Target, 1994, 2, 101-112. https://doi.org/10.3109/10611869409015898
- Goren, D.; Horowitz, A.T.; Tzemach, D.; Tarshish, M.; Zalipsky, S.; Gabizon, A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin. Cancer. Res., 2000, 6(5):1949-57. https://pubmed.ncbi.nlm.nih.gov/10815920
- Gosselin, M.A.; Lee, R.J. Folate receptor-targeted liposomes as vectors for therapeutic agents. Biotechnol. Annu. Rev., 2002, 8, 103-131. https://doi.org/10.1016/s1387-2656(02)08006-7
- Konda, S.D.; Aref, M.; Wang, S.; Brechbiel, M.; Wiener, E.C. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magma., 2001, 12, 104-113. https://doi.org/10.1007/bf02668091
- Moon, W.K.; Lin, Y.; O’Loughlin, T.; Tang, Y.; Kim, D.E.; Weissleder. R.; Tung, C.H. Enhanced tumor detection using a folate receptor-targeted near-infrared fluorochrome conjugate. Bioconjug. Chem., 2003, 14, 539-545. https://doi.org/10.1021/bc0340114
- Shukla, S.; Wu, G.; Chatterjee, M.; Yang, W.; Sekido, M.; Diop, L.A. Synthesis and biological evaluation of folate receptor-targeted boronated PAMAM dendrimers as potential agents for neutron capture therapy. Bioconjug. Chem., 2003, 14,158-167. https://doi.org/10.1021/bc025586o
- Lee, R.J.; Low, P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem., 1994, 269, 3198. https://doi.org/10.1016/S0021-9258(17)41848-5
- Ward, C.M. Folate-targeted non-viral DNA vectors for cancer gene therapy. Curr. Opin. Mol. Ther., 2000, 2, 182-187. https://pubmed.ncbi.nlm.nih.gov/11249640
- Reddy, J.A.; Abburi, C.; Hofland, H.; Howard, S.J.; Vlahov, I.; Wils, P.; Leamon, C.P. Folate-targeted, cationic liposome-mediated gene transfer into disseminated peritoneal tumors. Gene Ther., 2002, 9, 1542-1552. https://doi.org/10.1038/sj.gt.3301833
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev., 2002, 54, 675-693. https://doi.org/10.1016/s0169-409x(02)00042-x
- Reddy, J.A.; Clapp, D.W.; Low, P.S. Retargeting of viral vectors to the folate receptor endocytic pathway. J. Control. Release, 2001, 74, 77-82. https://doi.org/10.1016/s0168-3659(01)00316-9
- Turek, J.J.; Leamon, C.P.; Low, P.S. Endocytosis of folate–protein conjugates: ultrastructural localization in KB cells. J. Cell Sci., 1993, 106, 423-430. https://doi.org/10.1242/jcs.106.1.423
- Leamon, C.P.; Low, P.S. Cytotoxicity of momordin– folate conjugates in cultured human cells. J. Biol. Chem., 1992, 267, 24966-24971. https://doi.org/10.1016/s0021-9258(19)73992-1
- Jones, A.T.; Gumbleton, M.; Duncan, R. Understanding endocytic pathways and intracellular trafficking: a prerequisite for effective design of advanced drug delivery systems. Adv. Drug Deliv. Rev., 2003, 55, 1353-1357. https://doi.org/10.1016/j.addr.2003.07.002
- Antony, A.C. The biological chemistry of folate receptors. Blood, 1992, 79, 2807-2820.https://doi.org/10.1182/blood.v79.11.2807.2807
- Leamon, C.P.; Low, P.S. Delivery of macromolecules into living cells: A method that exploits folate receptor endocytosis. Proc. Natl. Acad. Sci. USA., 1991, 88, 5572-5576. https://doi.org/10.1073/pnas.88.13.5572
- Wileman, T.; Harding, C.; Stahl, P. Receptor mediated endocytosis. Biochem. J., 1985, 232, 1-14. https://doi.org/10.1042/bj2320001
- Smythe, E.; Warren, G. The mechanism of receptor-mediated endocytosis. Eur. J. Biochem., 1991, 202, 689. https://doi.org/10.1111/j.1432-1033.1991.tb16424.x
- Weitman, S.D.; Weinberg, A.G.; Coney, L.R.; Zurawski, V.; Jennings, D.S.; Kamen, B.A. Cellular localization of the folate receptor: Potential role in drug toxicity and folate homeostasis. Cancer Res., 1992, 52, 6708-6711. https://pubmed.ncbi.nlm.nih.gov/1330299
- Mattes, M.J.; Major, P.P.; Goldenberg, D.M.; Dion, A.S.; Hutter, R.V.; Klein, K.M. Patterns of antigen distribution in human carcinomas. Cancer Res., 1990, 50: 880s-884s. https://pubmed.ncbi.nlm.nih.gov/2297738
- Shen, F.; Ross, J.F.; Wang, X.; Ratnam, M. Identification of a novel folate receptor, a truncate receptor, and receptor type beta in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry, 1994, 33, 1209-1215. https://doi.org/10.1021/bi00171a021
- Sadasivan, E.; Rothenberg, S.P.; Costa, M.; Brink, L. Characterization of multiple forms of folate-binding protein from human leukemia cells. Biochem. Biophys. Acta., 1986, 882, 311-321. https://doi.org/10.1016/0304-4165(86)90253-9
- Ross, J.F.; Chaudhuri, P.K.; Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer, 1994, 73, 2432-2443. https://doi.org/10.1002/1097-0142(19940501)73:9%3C2432::aid-cncr2820730929%3E3.0.co;2-s
- Salazar, M.D.; Ratnam, M. The folate receptor: what does it promise in tissue targeted therapeutics? Cancer. Metastasis. Rev., 2007, 26, 141–152. https://doi.org/10.1007/s10555-007-9048-0
- Sega, E.I.; Low, P.S. Tumor detection using folate receptor-targeted imaging agents. Cancer. Metastasis. Rev., 2008, 27, 655–664. https://doi.org/10.1007/s10555-008-9155-6
- Luyckx, M.; Votino, R.; Squifflet, J.L.; Baurain, J.F. Profile of vintafolide (EC145) and its use in the treatment of platinum-resistant ovarian cancer. Int. J. Womens. Health., 2014, 6, 351–358. https://doi.org/10.2147/ijwh.s39696
- Choi, H.; Choi, S.R.; Zhou, R.; Kung, H.F.; Chen, I.W. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery. Academic. Radiology., 2004, 11, 996–1004. https://doi.org/10.1016/j.acra.2004.04.018
- Sonvico, F.; Mornet, S.; Vasseur, S.; Dubernet, C.; Jaillard. D.; Degrouard J. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconjugate Chemistry., 2005, 16, 1181–1188. https://doi.org/10.1021/bc050050z
- Santra, S.; Liesenfeld, B.; Dutta, D.; Chatel, D.; Batich, C.D.; Tan, W. Folate conjugated fluorescent silica nanoparticles for labeling neoplastic cells. Journal of Nanoscience and Nanotechnology, 2005, 5, 899–904. https://doi.org/10.1166/jnn.2005.146
- Bharali, D.J.; Luce, D.W.; Jayakumar, H.; Pudavar, H.E.; Prasad, P.N. Folate-receptor-mediated delivery of InP quantum dots for bioimaging using confocal and two-photon microscopy. Journal of the American Chemical Society, 2005, 127, 11364–11371. https://doi.org/10.1021/ja051455x
- Kam, N.W.; O’Connell, M.; Wisdom, J.A.; Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102, 11600–11605. https://doi.org/10.1073/pnas.0502680102
- Zheng, G.; Chen, J.; Li, H.; Glickson, J.D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102, 17757–17762. https://doi.org/10.1073/pnas.0508677102
- Polley, M.Y.; Freidlin, B.; Korn, E. L. Statistical and practical considerations for clinical evaluation of predictive biomarkers. J. Natl. Cancer. Inst., 2013, 105, 1677–1683. https://doi.org/10.1093/jnci/djt282
- Chen, Y.L.; Chang, M. C.; Huang, C.Y. Serous ovarian carcinoma patients with high alpha-folate receptor had reducing survival and cytotoxic chemo-response. Mol. Oncol., 2012, 6, 360–369. https://doi.org/10.1016/j.molonc.2011.11.010
- Bremer, R. E.; Scoggin, T.S.; Somers, E. B. Interobserver agreement and assay reproducibility of folate receptor alpha expression in lung adenocarcinoma: a prognostic marker and potential therapeutic target. Arch. Pathol. Lab. Med., 2013, 137, 1747–1752. https://doi.org/10.5858/arpa.2013-0039-oa
- Hartmann, L.C.; Keeney, G. L.; Lingle, W. L. Folate receptor overexpression is associated with poor outcome in breast cancer. Int. J. Cancer., 2007, 121, 938–942. https://doi.org/10.1002/ijc.22811
- Parker, N.; Turk, M. J.; Westrick, E. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem., 2005, 338, 284–293. https://doi.org/10.1016/j.ab.2004.12.026
- Basal, E.; Eghbali-Fatourechi, G. Z.; Kalli, K. R. Functional folate receptor alpha is elevated in the blood of ovarian cancer patients. PLoS One., 2009, 4, e6292. https://doi.org/10.1371/journal.pone.0006292
- O’Shannessy, D. J.; Somers, E. B.; Palmer, L. M. Serum folate receptor alpha, mesothelin and megakaryocyte potentiating factor in ovarian cancer: association to disease stage and grade and comparison to CA125 and HE4. J. Ovarian. Res., 2013, 6, 29. https://doi.org/10.1186/1757-2215-6-29
- O’Shannessy, D. J.; Gustavson, M.; Chandrasekaran, L. K. Prognostic significance of FRA expression in epithelial cancers using AQUA® technology. Biomark Med., 2013, 7, 933–946. https://doi.org/10.2217/bmm.13.85
- Iwakiri, S.; Sonobe, M.; Nagai, S. Expression status of folate receptor alpha is significantly correlated with prognosis in non-small-cell lung cancers. Ann. Surg. Oncol., 2008, 15, 889–899. https://doi.org/10.1245/s10434-007-9755-3
- Gerlinger, M.; Rowan, A. J.; Horswell, S. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med., 2012, 366, 883–892. https://doi.org/10.1056/nejmoa1113205
- Almendro, V.; Marusyk, A.; Polyak, K. Cellular heterogeneity and molecular evolution in cancer. Annu. Rev. Pathol., 2013, 8, 277–302. https://doi.org/10.1146/annurev-pathol-020712-163923
- Schwarz, R. F.; Ng, C. K.; Cooke, S.L. Spatial and temporal heterogeneity in high grade serous ovarian cancer: a phylogenetic analysis. PLoS Med., 2015, 12, e1001789. https://doi.org/10.1371/journal.pmed.1001789
- Leamon, C. P.; Parker, M. A.; Vlahov, I.R. Synthesis and biological evaluation of EC20: a new folate-derived, 99mTc-based radiopharmaceutical. Bio. Conjug. Chem., 2002, 13: 1200–1210. https://doi.org/10.1021/bc0200430
- Fisher, R.E.; Siegel, B. A.; Edell, S.L. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J. Nucl. Med., 2008, 49, 899–906. https://doi.org/10.2967/jnumed.107.049478
- Mathias, C.J.; Wang, S.; Low, P. S. Receptor-mediated targeting of 67Gadeferoxamine-folate to folate-receptor-positive human KB tumor xenografts. Nucl. Med. Biol., 1999, 26, 23–25. https://doi.org/10.1016/s0969-8051(98)00076-6
- Wang, S.; Luo, J.; Lantrip, D. A. Design and synthesis of [111In] DTPA-folate for use as a tumor-targeted radiopharmaceutical. Bio. conjugate. Chem., 1997, 8, 673–679. https://doi.org/10.1021/bc9701297
- Guo, W.; Hinkle, G. H.; Lee, R.J. 99mTc-HYNIC-folate: a novel receptor-based targeted radiopharmaceutical for tumor imaging. J. Nucl. Med., 1999, 40, 1563–1569. https://pubmed.ncbi.nlm.nih.gov/10492380
- Siegel, B. A.; Dehdashti, F.; Mutch, D. G. Evaluation of 111In-DTPA-folate as a receptor-targeted diagnostic agent for ovarian cancer: initial clinical results. J. Nucl. Med., 2003, 44, 700–707. https://pubmed.ncbi.nlm.nih.gov/12732670
- Van, Dam.; G, M.; Themelis, G.; Crane, L. M. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first inhuman results. Nat. Med., 2011, 17, 1315–1319. https://doi.org/10.1038/nm.2472
- AlJammaz, I.; Al-Otaibi, B.; Al-Rumayan, F. Development and preclinical evaluation of new (124) I-folate conjugates for PET imaging of folate receptor positive tumors. Nucl. Med. Biol., 2014, 41, 457–463. https://doi.org/10.1016/j.nucmedbio.2014.03.013
- Edelman, M. J.; Bonomi, P.; Harb, W. Co-development of a folate receptor targeted drug conjugate vintafolide (EC145) and a folate receptor targeted imaging agent 99mTc-etarfolatide (EC20) in the treatment of advanced adenocarcinoma NSCLC. J. Thorac. Oncol., 2012, 7, S63. https://www.researchgate.net/publication/296033927
- Morris, R. T.; Joyrich, R. N.; Naumann, R. W. Phase II study of treatment of advanced ovarian cancer with folate-receptor-targeted therapeutic (vintafolide) and companion SPECT-based imaging agent (99mTc-Tetarfolatide). Ann. Oncol., 2014, 25, 852–858. https://doi.org/10.1093/annonc/mdu024
- Konda, S.D. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: work in progress. Invest. Radiol., 2000, 35, 50–57. https://doi.org/10.1097/00004424-200001000-00006
- Meier, R. Breast cancers: MR imaging of folate receptor expression with the folate-specific nanoparticle P1133. Radiology., 2010, 255, 527–535. https://doi.org/10.1148/radiol.10090050
- Ao, L. A folate-integrated magnetic polymer micelle for MRI and dual targeted drug delivery. Nanoscale., 2014, 6, 10710–10716. https://doi.org/10.1039/c4nr02484b
- Brand, C. Leveraging PET to image folate receptor α therapy of an antibody-drug conjugate. EJNMMI Res., 2018, 8, 87. https://doi.org/10.1186/s13550-018-0437-x
- Kennedy, M.D. Optical imaging of metastatic tumors using a folate-targeted fluorescent probe. J. Biomed. Opt., 2003, 8, 636–641. https://doi.org/10.1117/1.1609453
- Horowitz, N.S. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced stage ovarian cancer: an analysis of GOG 182. J. Clin. Oncol., 2015, 233, 937–943. https://doi.org/10.1200/jco.2014.56.3106
- Martin, L.W. Detection of occult micrometastases in patients with clinical stage I non-small-cell lung cancer: a prospective analysis of mature results of CALGB 9761 (Alliance). J. Clin. Oncol., 2016, 34, 1484–1491. https://doi.org/10.1200/jco.2015.63.4543
- De, La. Cruz. L. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann. Surg. Oncol., 2016, 23, 3247–3258. https://doi.org/10.1245/s10434-016-5313-1
Rights and Permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party materials in this article are included in the articles Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Creative Commons This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. You are not required to obtain permission to reuse this article. To request permission for a type of use not listed, please contact Biotechnology Kiosk.