3D Tissue Constructs from the Ground Up for Game Changing New Applications in Tissue Engineering and Regenerative Medicine
Pallavi Tripathi1, Sunny O. Abarikwu2*
1Intertek Pharmaceutical Services Manchester, Manchester, UK.
2Department of Biochemistry, University of Port Harcourt Choba, Nigeria.
Abstract
In view of the very expensive modern healthcare system, sudden loss or failure of organs and tissues could pose a very difficult and costly medical problem to patients. Further, the limited supply of organs globally that a patient can afford for replacement in the event of an organ failure makes the problem even more challenging and complicated. These medical and healthcare challenges have triggered research and developments into tissue engineering to advance the field of regenerative medicine. Especially, the research focus has been on the design, development and optimization of a cell-scaffold-microenvironment to promote the regeneration of various types of tissue including skin, cartilage, bone, tendon and cardiac tissue, to name a few. Studies have been undertaken to produce functional three-dimensional (3D) tissue substitutes or constructs that are based on bio scaffolds from the ground up. To this end, bioprinting strategies have been considered for fabrication of complex 3D functional living tissues or artificial organs. Here, we describe some notable advances in laser bioprinting enabled tissue engineering, which is a rapidly emerging field in 3D biofabrication technology for applications in regenerative medicine.
Keywords: 3D bioprinting, laser assisted bioprinting, tissue engineering, artificial organs
*Corresponding author: Sunny O. Abarikwu
E-mail address: sunny.abarikwu@uniport.edu.ng
DOI: https://doi.org/10.37756/bk.22.4.3.2
Article type: Feature
Received: January 5, 2022
Revised: January 29, 2022
Accepted: February 5, 2022
Please cite this article as: Tripathi P and Abarikwu S, 3D Tissue Constructs from the Ground Up for Game Changing New Applications in Tissue Engineering and Regenerative Medicine, Biotechnol. kiosk, Vol 4, Issue 3, PP: 17-31 (2022); DOI: https://doi.org/10.37756/bk.22.4.3.2
Introduction
The sudden loss or failure of organs and tissues is a serious medical condition. Failure of organs is widely recognized as a difficult and costly problem in modern healthcare. Further, the patient’s problem becomes even more challenging in view of the limited supply of organs globally. To address these issues, there has been a steady growth of research on tissue engineering that especially involves the fabrication and design of 3-dimensional (3D) artificial scaffolds and a cell-scaffold-microenvironment that mimics human tissue for the development of artificial organs. The goal is to promote the regeneration of various types of tissue including skin, liver, cartilage, bone, tendon and cardiac tissue, to name a few [1-5].
3D tissue constructs are usually built by a combination of biocompatible and bioactive biomaterials with/or without cells and bioactive factors. Such constructs are developed from the ground up with the aim to replace or sustain the regeneration of tissues. Studies have shown scaffolds as the key element for tissue regeneration, which can be leveraged to provide the necessary mechanical support and a physical structure for the transplanted cells to attach and grow. This can be achieved together with maintaining their physiological functions. To obtain best performance, the key parameters to control are cell density along with cell spatial 3D organization that govern cell behavior and fate [6-9].
With respect to scaffolds, a bone scaffold for tissue engineering is of enormous interest due to the ever growing demand of new biomedical implants. To be able to generate fully functional properties, bone scaffolds need to have favorable biocompatibility or cytocompatibility. This is essential to provide a surface for cells that can adhere, proliferate, differentiate and secrete extracellular matrix (ECM). Further, it has been shown that cell adhesion and migration, vascularization and new tissue ingrowth are influenced by pore size and interconnectivity. All these factors imply that a high-performance scaffold must simultaneously support the growth of different cell types and tissues, each with specific mechanical properties, chemical gradients, cell populations, and geometric structures. Several conventional fabrication methods including electrospinning, fiber deposition, freeze-drying, gas foaming, and salt leaching have been considered for manufacturing 3D scaffolds. However, these methods have limitations that do not allow precise control of internal structural features and topology. Especially, current traditional methods have complex design constraints that restrict the applicability of these methods particularly in serious medical conditions that include repairing clinically relevant injuries, organs, and other complex tissues. This has motivated researchers to develop new techniques for the accurate fabrication of multifunctional scaffolds. [1, 10-14].
Bioprinting is an emerging technique for 3D tissue constructs from the ground up. The technique employs biomaterials, cells, and/or bioink to fabricate prospective scaffolds to mirror the structural, compositional, and functional aspects of various human tissues. Several bioprinting methods including inkjet-based bioprinting, pressure-assisted bioprinting, and laser-assisted bioprinting have been employed for applications in regenerative medicine. The fabricated scaffolds have been characterized based on biocompatibility, cellular microenvironment, cell proliferation, vitality, and morphology [15]. Among other bioprinting methods, laser bioprinting is considered most promising for 3D tissue engineering and for fabrication of multifunctional bioscaffolds. Here, we will present some notable advances in laser bioprinting for 3D tissue constructs for applications in regenerative medicine.
Bioprinting Strategies
Recent advances in biofabrication have led to the development of Bioprinting, which is a rapidly emerging 3D biofabrication technology. Bioprinting is employed to precisely dispense cell-laden biomaterials for the construction of complex 3D functional living tissues or artificial organs. Figure 1 shows a typical 3D printing technical route, which is aided by 3D imaging software [1]. 3D bioprinting is considered a transformative technology because it allows more accurate personalized manufacturing of biomedical devices that need to be created to the patient’s own specifications. In addition, bioprinting technology has a vast potential in a range of other applications that include creating more accurate non-biologic and biologic research models for research purposes, for example, spatial and temporal trauma in cancer research [1].
Recent studies have suggested that the design and manufacturing of living tissues and organs by 3D bioprinting could be leveraged in future to be implanted into patients safely with no side effects. In view of the enormous promise of bioprinting, researchers across the globe are currently exploring this technique to pattern cells or fabricate several different tissues and a whole host of functional organs, for example, blood vessels or cardiac patches, just to name a few. Bioprinting strategies are still in its early stages of developments. It is believed that with further advances, the versatile bioprinting may address the issues of growing organ shortage in the world by providing a high-throughput method for cell patterning at the micrometer scale for broad biomedical engineering applications. However, current approaches have to overcome technical challenges that include high-resolution cell deposition, controlled cell distributions, vascularization, and innervation within complex 3D construct tissues to achieve the desired potential of 3D bioprinting [16-21].
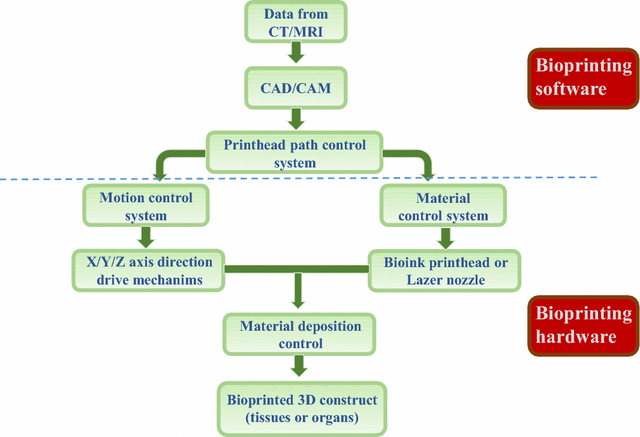
Figure 1: A typical workflow is shown for the general process of 3D bioprinting [Source: Journal of Translational Medicine (2016)].
Laser Assisted Bioprinting: A Potential Game Changing Biofabrication Technology for 3D Tissue Constructs
To address the technical challenges in 3D bioprinting, recent developments in laser based bioprinting has been shown very promising in 3D tissue constructs from the ground up. Laser based 3D printing allows fabrication of tissue-like structures that have the exact physiological functionality of their native counterparts. In addition, it allows printing cells and liquid materials with a cell- or picoliter-level resolution that is believed to be a game changer in 3D tissue engineering and fabrication of artificial organs. Further, this technology can enable study on cellular interactions and to fabricate cell‐based biosensors due to its capacity to spatially control cell position and cell density. It has additional advantages such as automation, reproducibility, and high throughput. Thus, it makes the process compatible with the industrial scale fabrication of 3D constructs of physiologically relevant sizes [22, 23].
The laser based bioprinting process is essentially a direct-write method that allows droplet deposition of cells or biomaterials within a fluidic phase. With respect to the pace of the process, a MHz range speed can be obtained, where a near infrared pulsed laser beam is employed that is coupled to a scanning mirror and a focusing system. Further, this process involves a transparent substrate, which is usually coated with a thin layer of laser-absorbing material along with a second thicker layer of biomaterial that is made of hydrogel with embedded cells to be printed [24, 25]. Laser pulses are then focused into the laser-absorbing layer (Figure 2) [26]. This step is often referred to Dynamic-Release-Layer (DRL), which corresponds to the underlying process of vaporization in the focal region that generate a vapor bubble. This bubble then expands by vapor pressure that subsequently propels the adjacent biomaterial forward. This eventually helps it to get deposited as a droplet at a predefined position on a collector slide. Researchers have shown that the high energy created by the incidence of the laser beam can create a cavitation that can eventually propel a microdroplet, containing cells, towards the receiving substrate. Such a configuration can be a 2D support or an exposed 3D in-vivo tissue [24-26].
Another advantage of laser based bioprinting is its nozzle-free hardware. This allows avoiding the problem of cell clogging and thus helps overcoming the issues encountered in other bioprinting approaches. This can be achieved while providing the desired control on the density and microscale distribution of cells along with their viability and to attain higher speeds of deposition. Additionally, the technology enables an unprecedented printing precision at the micrometer scale. This provides the opportunity to achieve an ultimate control over cell organization for 3D tissue constructs from the ground up for a number of personalized medicine and healthcare applications [25].
In the following sections, we will take a look at some of the important applications of laser assisted 3D bioprinting.
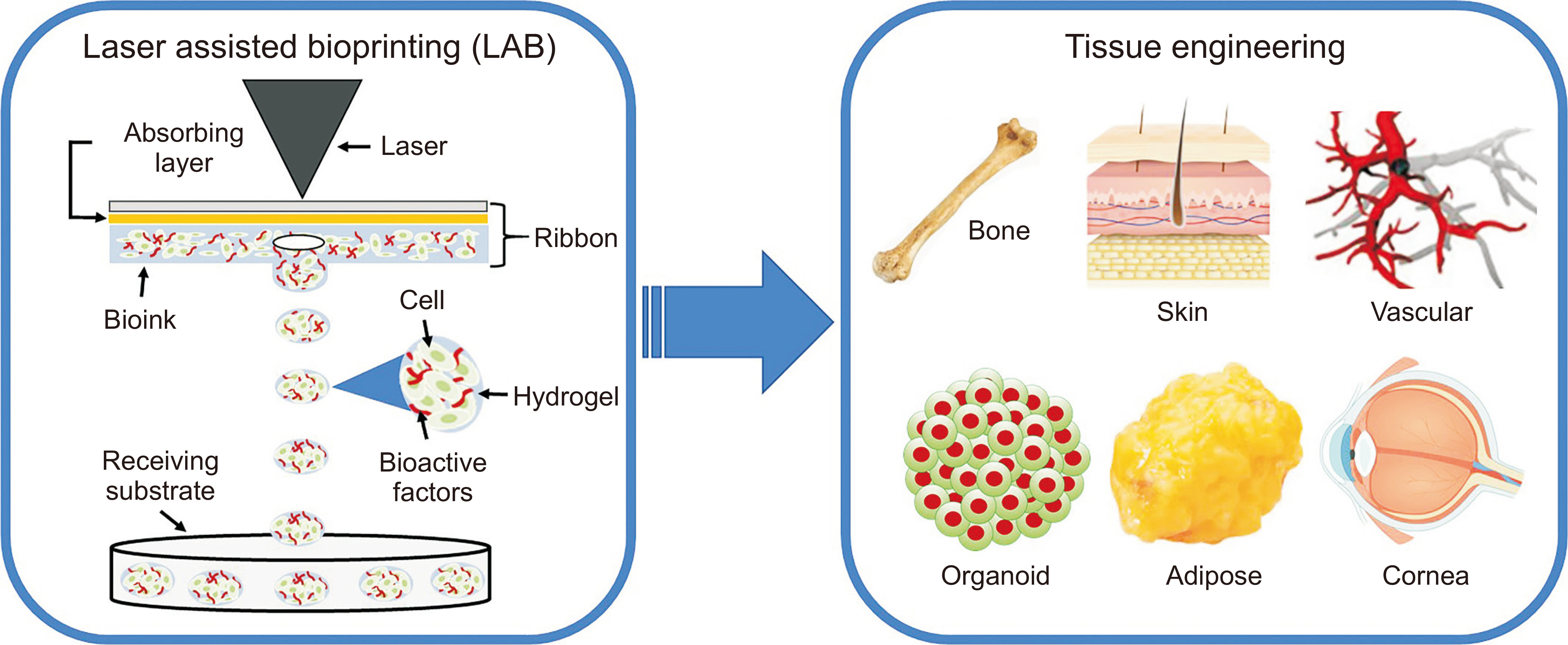
Figure 2: Schematic depiction of different parts of laser assisted bioprinting along with various tissue engineering applications of fabricated different 3D tissue constructs [Source: Med Lasers (2021)].
Guided Regeneration of In-Vivo Bone Tissue by Laser Bioprinting of Mesenchymal Stromal Cells
Mesenchymal Stromal Cells ‘MSCs’ are known as multipotent progenitor cells that have the capacity to differentiate into a variety of cell types including osteoblasts, adipocytes, chondrocytes, tenocytes and skeletal myocytes. Additionally, these cells also have immunomodulatory properties that can be purified from different tissues (e.g. bone marrow, adipose tissue, umbilical cord). Studies have shown that MSCs have the capacity to secrete protective biological factors. This attribute makes them as one of the most suitable cell source for tissue regeneration approaches [27, 28].
In a notable study, researchers combined the printing of hydroxyapatite (HA) with MSCs, D1 cell line and investigated the impact of two different cell-printing geometries that have distinctive cellular repartitions (disc or ring) on bone repair capabilities [25]. Subsequently, two HA-collagen disks were printed before and after the cellularized ink printing in order to confine the printed cell spots to the calvaria defect. This was also done to provide an osteoconductive matrix to the printed cells. Figure 3 shows a schematic representation of the in-vivo laser based bioprinting geometries that were tested with a ring (A1) with external and internal diameter of 3 and 2.1 mm, respectively, and a disk (B1) with 2 mm diameter [25].
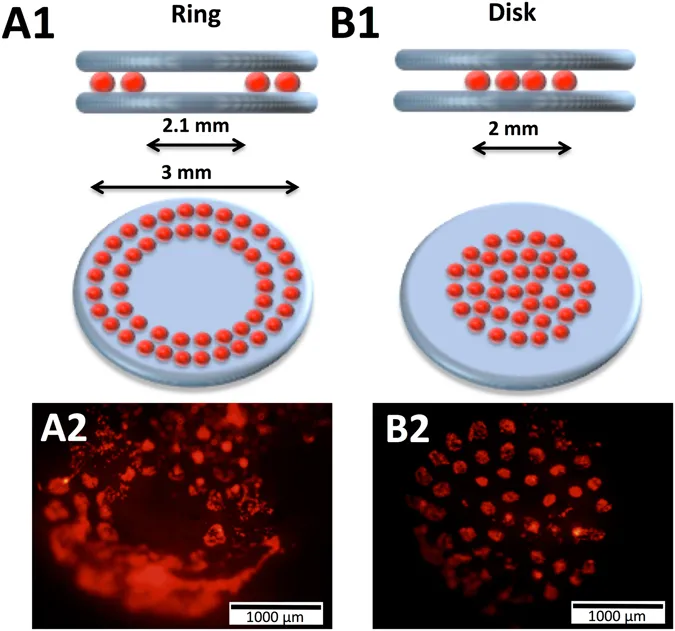
Figure 3: Laser assisted bioprinting for in-situ printing of mesenchymal stromal cells for in-vivo bone regeneration applications (it shows a ring (A1) with and a disk (B1) along with representative fluorescence images of ring (A2) and disk (B2) printed cells inside the calvaria defect in mice) [Source: Scientific Reports, (2017)].
Two layers of HA-collagen ink were printed underneath and over the cellularized ink layer in this study that employed two geometries. Figure 3 shows representative fluorescence images of ring (A2) and disk (B2) printed tomato-positive (D1) cells inside the calvaria defect in mice that were obtained immediately after printing. Further, researchers observed a significant increase in terms of bone formation in the case of HA collagen material and with D1 cells in a disk geometry post printing. Thus, a key finding was reported that involved testing different cell printing geometries and different cellular arrangements that impacted bone tissue regeneration differently. This work paves the way to new avenues on the development of in-situ laser bioprinting strategies for the building of 3D tissue constructs from the ground up [25].
Laser Bioprinting for Cardiovascular Repair/Regeneration and Pharmacological Applications
Cardiovascular disease (CVD) is thought to be a major cause of morbidity and mortality worldwide. CVD is associated with serious medical conditions such as congenital heart disease, acute coronary syndrome, hypertension, and arrhythmias that emanate from the faulty cardiovascular system. This fatal disease account for >17.5 million deaths per year, and that is estimated to increase to 23.6 million by 2030 [29-33]. To gain insights into CVD, it is critical to understand the cardiovascular system, which is a very important part of human body that includes the heart, blood vessels (arteries, veins, arteriovenous shunts, and capillaries), and lymphatic vessels. It is known that the cardiovascular system corresponds to a closed loop transport system that carries blood and lymph for circulation throughout the body. Laser based bioprinting for cardiovascular repair and regeneration is currently of huge interest that offers hope in providing new solutions to these very challenging and complicated problems in future healthcare sector dealing with CVD [33].
While the bioprinting technique for cardiac tissue constructs is still in its early stages, It is generally believed that the laser based 3D bioprinting would be a feasible approach in the future to produce a robust, and physiologically relevant, cardiac model. This could be realized by replicating in-vivo tissue composition, geometry, and complexity of the cardiovascular system, in general. 3D bioprinting can be leveraged to create complicated cardiovascular implants with biomimetic features that are capable of recapitulating both the native physiochemical and biomechanical characteristics of the cardiovascular system. Ongoing studies on micro-physiological models of the 3D bioprinted heart have focused mostly on generating the myocardium, valve, and vessels. Additionally, researchers are also focusing on the design and development of a bioartifical heart valve that mimics the structural and the functional aspect of a native valve for use as an implantable device. To achieve the goals, one viable clinically attractive approach being considered is in-situ heart valve tissue engineering that employs cell-free synthetic, biodegradable scaffolds to create living valves right inside the heart of a patient. Figure 4 schematically shows notable advances in 3D bioprinting cardiovascular tissues/models for regeneration and pharmacological modeling applications [33].
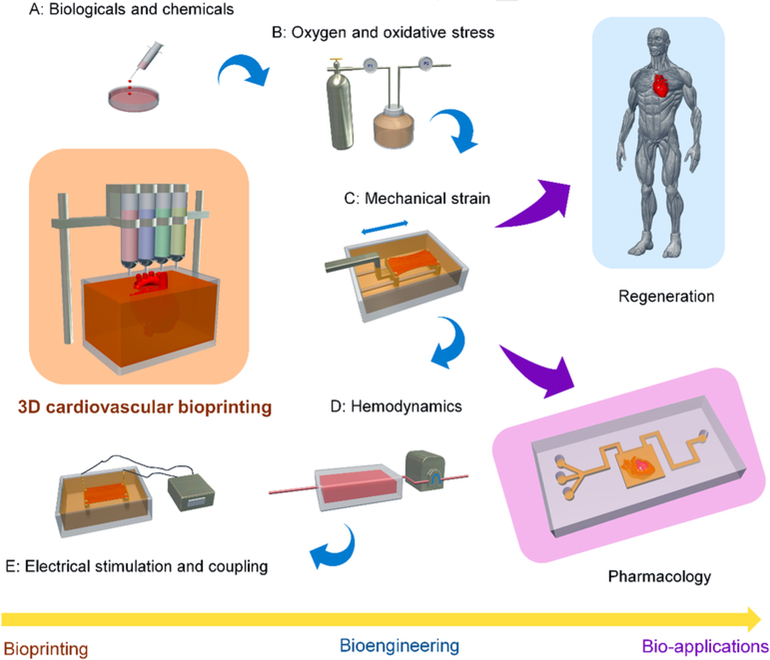
Figure 4: Schematic diagram showing techniques of 3D cardiovascular bioprinting along with bioengineering methods, and bio-applications in regenerative medicine and pharmacology [Source: Adv. Drug Deliv. Rev. (2018)].
In an earlier study, researchers showed that mesenchymal stem cells (MSC) could inhibit apoptosis of endothelial cells in hypoxic condition, increase their survival, and stimulate the angiogenesis process. To investigate in detail, researchers employed Laser-Induced-Forward-Transfer (LIFT) cell printing technique and prepared a cardiac patch seeded with human umbilical vein endothelial cells (HUVEC) and human MSC (hMSC) in a defined pattern for cardiac regeneration (Figure 5) [8].
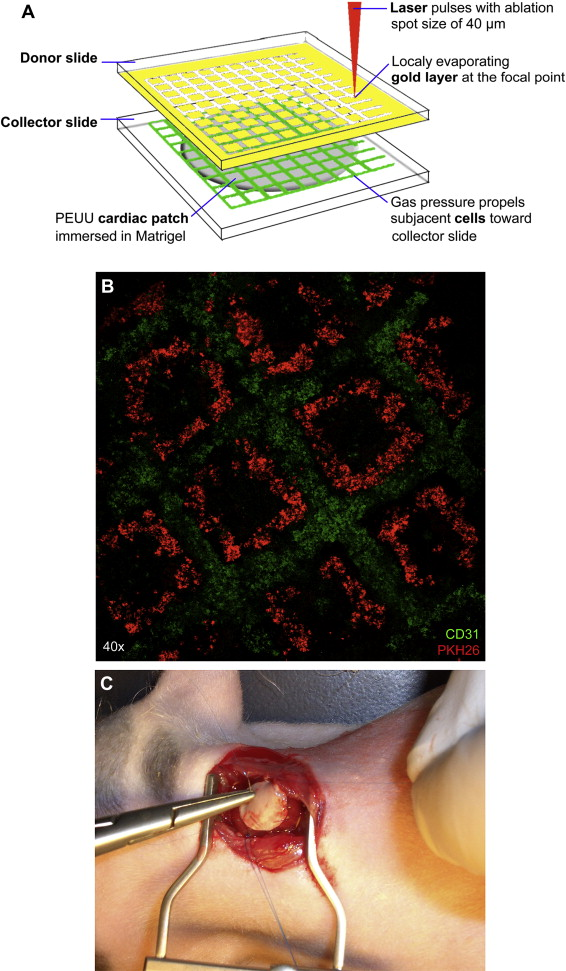
Figure 5: (A) Schematic representation of laser based cell printing showing cardiac patch manufacturing. (B) Fluorescent image of micropatterned hMSCs (red) and hUVECs (green). (C) Cardiac patch implanted into rat myocardium [Source: Biomaterials (2011)].
In their study, researchers seeded HUVEC and hMSC in a defined pattern on a Polyester urethane urea (PEUU) cardiac patch. Subsequently, they cultivated cardiac patches in-vitro and transplanted in-vivo to the infarcted zone of rat hearts. It was demonstrated that LIFT-derived cell seeding pattern modified growth characteristics of co-cultured HUVEC and hMSC. This resulted in an increased vessel formation. In this study, major functional improvement of infarcted hearts was found following transplantation of a LIFT-tissue engineered cardiac patch. Researchers concluded that LIFT-based Tissue Engineering of cardiac patches for the treatment of myocardial infarction might improve wound healing and functional preservation [34].
Laser Bioprinting of Skin Constructs: Cure for Difficult-To-Repair Extensive Burns and Full-Thickness Skin Wounds
It is widely recognized that repairing extensive burns and full-thickness skin wounds is a significant medical challenge. This is especially true considering deep damage to the skin that happens in these serious medical conditions. The existing skin graft technology based on Autologous Split-thickness Skin Graft (ASSG) is limited for applications due to the shortage of donor skin tissues, which is a serious problem. Skin bioprinting is considered very promising that may provide a novel alternative to ASSG therapy. It is believed that laser assisted bioprinting can address this problem that can provide a potential solution. The main advantage of employing laser based skin bioprinting is the availability of skin constructs fabricated by using in-vitro expanded cells from skin biopsy that would mitigate the problem of shortage of donor sites encountered in ASSG. Studies have suggested the possibility of bioprinting of skin tissue constructs that can lead to the development of skin equivalents for wound healing therapy. To this end, researchers have fabricated skin constructs using biomaterial scaffolds with or without cells to create skin tissues that are suitable for transplantation. In this process, skin tissues are usually collected from patients in skin bioprinting by skin biopsy. This can be then cultured in-vitro to obtain enough number of cells. Subsequently, cultured skin cells are mixed with biomaterials and then delivered to a 3D bioprinter for fabrication of customized skin as prescribed by the patient’s specific requirements [34-37].
Ongoing studies have focused on to address technological challenges for the development of bio-mimetic functional skin for clinical applications. For example, a related study conducted on laser based bioprinting of skin tissue demonstrated direct printing of amniotic fluid-derived stem cells (AFSCs) onto full-thickness skin wounds (2 cm × 2 cm) of nu/nu mice using a pressure-driven, computer controlled bioprinting device (Figure 6) [38]. In this study, AFSCs and bone marrow-derived mesenchymal stem cells were suspended in fibrin-collagen gel and mixed with the thrombin solution (a crosslinking agent) that were then printed onto the wound site. Researchers then employed the bioprinter that was used to deposit two layers of a fibrin-collagen gel by depositing a layer of thrombin, a layer of fibrinogen/collagen, a second layer of thrombin, a second layer of fibrinogen/collagen, and a final layer of thrombin (Figure 6) [38].
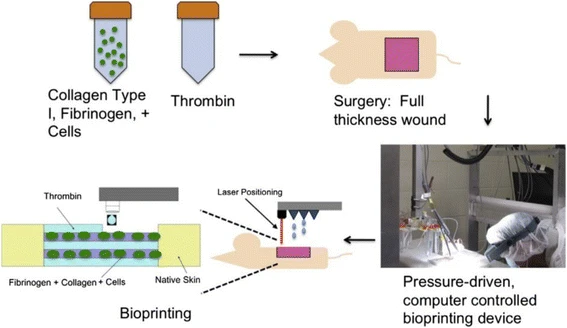
Figure 6: A schematic depiction of the approach of in-situ laser based bioprinting of artificial skin [Source: Burns & Trauma (2018)].
Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks
The cornea is the transparent anterior part of the eye, which is a critical part for vision. Corneal blindness due to trauma or diseases is a serious ophthalmic condition that affects millions of people worldwide. Therefore, a significant volume of research has been devoted to meet the high demand for developing methods to produce more native-like 3D corneal structures [39-41]. In a recent study, researchers produced 3D cornea-mimicking tissues using human stem cells and laser-assisted bioprinting (LaBP). Researchers used human embryonic stem cell derived limbal epithelial stem cells (hESC-LESC) as a cell source for printing epithelium-mimicking structures. Whereas human adipose tissue derived stem cells (hASCs) were used for constructing layered stroma-mimicking structures. It was shown that the development and optimization of functional bioinks was a crucial step towards successful bioprinting of 3D corneal structures (Figure 7) [42]. Further, in this study, recombinant human laminin and human sourced collagen I served as the bases for the functional bioinks. Researchers employed two previously established LaBP setups based on laser induced forward transfer, with different laser wavelengths and appropriate absorption layers [42].
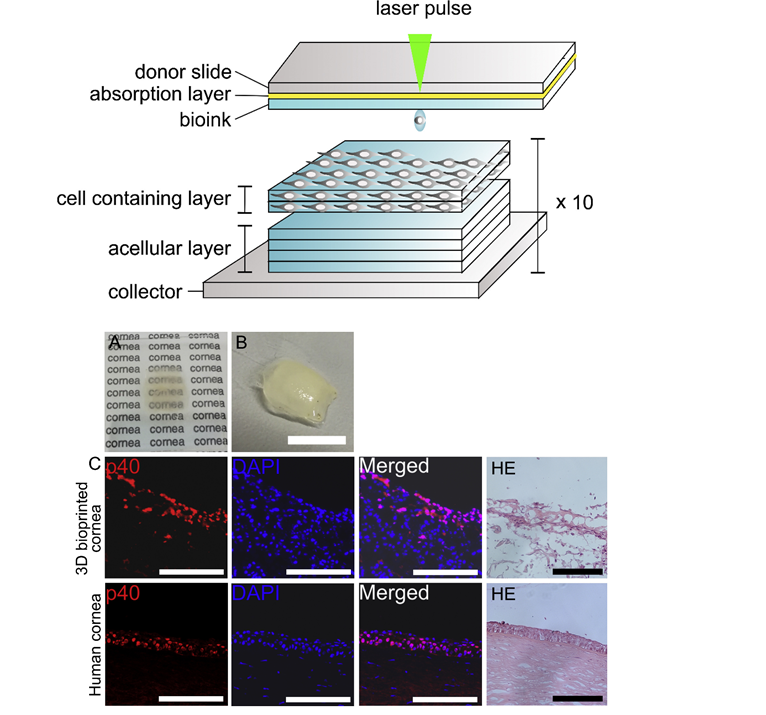
Figure 7: (Top) Schematic diagram of the laser-assisted bioprinting system and printing of the 3D stromal mimicking structures. (Bottom) (A-C) a proof-of-concept to fabricate tissue-engineered cornea using both investigated human stem cell types (the bioprinted 3D cornea fabricated on PET substrate showing moderate transparency, while printing on non-transparent Matriderm® substrate was required to avoid shrinkage of the structure during culture). It also shows comparison between the 3D bioprinted corneal tissue and the native human cornea (Hematoxylin and eosin (HE)-staining shows the structure of the bioprinted tissue) [Source: Biomaterials (2018)].
In this study, researchers bioprinted three types of corneal structures that included stratified corneal epithelium using hESC-LESCs, lamellar corneal stroma using alternating acellular layers of bioink and layers with hASCs, and finally structures with both a stromal and epithelial part. The printed constructs were then evaluated for their microstructure, cell viability and proliferation, and key protein expression (Ki67, p63α, p40, CK3, CK15, collagen type I, VWF). Further, the 3D printed stromal constructs were also implanted into porcine corneal organ cultures that showed both cell types maintained good viability after printing. This was the first study to demonstrate the feasibility of 3D LaBP for corneal applications using human stem cells and successful fabrication of layered 3D bioprinted tissues that mimicked the structure of the native corneal tissue [42].
Conclusion
We have presented an overview of laser based bioprinting of 3D tissue constructs, which is considered a very promising area of current research and developments into tissue engineering and regenerative medicine. Laser based 3D bioprinting is a multidisciplinary field that is bringing together experts in cell biology, mechanical engineering, biotechnology, biomaterials science and laser science and engineering, to name a few. Studies conducted so far have shown tremendous potential of stem-cell bioprinting as a source for renewable human tissue that offers a technological breakthrough in creating organs that are biocompatible. In this regard, early research on various types of artificial tissues have shown promise using bioprinted stem cells from liver to brain.
More studies will probably be needed in the field of personalized 3D printing technology. One specific area is fabricating artificial pancreas for diabetic patients. It is believed that future studies will focus on printing micro-organs that include pancreas islet tissues that function in the absence of the complete pancreas structure. This will benefit hundreds of millions of diabetic patients around the globe. However, the challenge will be to address a series of regulatory hurdles in the specified printed product. We anticipate a transition of printing adult stem cells to clinical trials and eventually to medical industry in the near future. The future of laser assisted 3D bioprinting in tissue engineering looks very promising.
References
[1] Li J, Chen M, Fan X, Zhou H, Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med 2016, 14:271. DOI: https://doi.org/10.1186/s12967-016-1028-0
[2] Thankam FG, Agrawal DK, Infarct Zone: a Novel Platform for Exosome Trade in Cardiac Tissue Regeneration. J Cardiovasc Transl Res 2020, 13(5):686-701. DOI: https://doi.org/10.1007/s12265-019-09952-8
[3] Zhang Q, Johnson JA, Dunne LW, Chen Y, Iyyanki T, Wu Y, Chang EI, Branch-Brooks CD, Robb GL, Butler CE, Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater 2016, 35:166-84. DOI: https://doi.org/10.1016/j.actbio.2016.02.017
[4] Chang RC, Emami K, Jeevarajan A, Wu H, Sun W, Microprinting of liver micro-organ for drug metabolism study. Methods Mol Biol 2011, 671:219-38. DOI: https://doi.org/10.1007/978-1-59745-551-0_13
[5] Thankam FG, Muthu J. Alginate-polyester comacromer based hydrogels as physiochemically and biologically favorable entities for cardiac tissue engineering. J Colloid Interface Sci 2015, 457:52-61. DOI: https://doi.org/10.1016/j.jcis.2015.06.034
[6] Lee J, Cuddihy MJ, Kotov NA, Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 2008, 14(1):61-86. DOI: https://doi.org/10.1089/teb.2007.0150
[7] Hollister SJ, Maddox RD, Taboas JM, Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2002, 23(20):4095-103. DOI: https://doi.org/10.1016/s0142-9612(02)00148-5
[8] Futrega K, Palmer JS, Kinney M, Lott WB, Ungrin MD, Zandstra PW, Doran MR, The microwell-mesh: A novel device and protocol for the high throughput manufacturing of cartilage microtissues. Biomaterials 2015, 62:1-12. DOI: https://doi.org/10.1016/j.biomaterials.2015.05.013
[9] Lee CH, Lee FY, Tarafder S, Kao K, Jun Y, Yang G, Mao JJ, Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest 2015, 125(7):2690-701. DOI: https://doi.org/10.1172/JCI81589
[10] Shamaz BH, Anitha A, Vijayamohan M, Kuttappan S, Nair S, Nair MB, Relevance of fiber integrated gelatin-nanohydroxyapatite composite scaffold for bone tissue regeneration. Nanotechnology 2015, 26(40):405101. DOI: https://doi.org/10.1088/0957-4484/26/40/405101
[11] Rouwkema J, Rivron NC, van Blitterswijk CA, Vascularization in tissue engineering. Trends Biotechnol 2008, 26(8):434-41. DOI: https://doi.org/10.1016/j.tibtech.2008.04.009
[12] Jones AC, Arns CH, Hutmacher DW, Milthorpe BK, Sheppard AP, Knackstedt MA, The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30(7):1440-51. doi: https://doi.org/10.1016/j.biomaterials.2008.10.056
[13] Leong KF, Chua CK, Sudarmadji N, Yeong WY, Engineering functionally graded tissue engineering scaffolds. J Mech Behav Biomed Mater. 2008, 1(2):140-52. DOI: https://doi.org/10.1016/j.jmbbm.2007.11.002
[14] Moroni L, de Wijn JR, van Blitterswijk CA, 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27(7):974-85. DOI: https://doi.org/10.1016/j.biomaterials.2005.07.023
[15] Ishack S, Lipner SR, A Review of 3-Dimensional Skin Bioprinting Techniques: Applications, Approaches, and Trends. Dermatol Surg 2020, 46(12):1500-1505. DOI: https://doi.org/10.1097/DSS.0000000000002378
[16] Zhang S, Wang H, Current Progress in 3D Bioprinting of Tissue Analogs. SLAS Technol 2019, 24(1):70-78. DOI: https://doi.org/10.1177/2472630318799971
[17] Shafiee A, Atala A, Printing Technologies for Medical Applications. Trends Mol Med 2016, 22(3):254-265. DOI: https://doi.org/10.1016/j.molmed.2016.01.003
[18] Li H, Fan W, Zhu X, Three-dimensional printing: The potential technology widely used in medical fields. J Biomed Mater Res A 2020, 108(11):2217-2229. DOI: https://doi.org/10.1002/jbm.a.36979
[19] Shapira A, Dvir T, 3D Tissue and Organ Printing-Hope and Reality. Adv Sci (Weinh) 2021, 8(10):2003751. DOI: https://doi.org/10.1002/advs.202003751
[20] Christian Mandrycky, Zongjie Wang, Keekyoung Kim Deok-Ho Kim, 3D bioprinting for engineering complex tissues, Biotechnology Advances, 34, 422 (2016).
[21] Ostrovidov S, Salehi S, Costantini M, Suthiwanich K, Ebrahimi M, Sadeghian RB, Fujie T, Shi X, Cannata S, Gargioli C, Tamayol A, Dokmeci MR, Orive G, Swieszkowski W, Khademhosseini A, 3D Bioprinting in Skeletal Muscle Tissue Engineering. Small 2019, 15(24):e1805530. DOI: https://doi.org/10.1002/smll.201805530
[22] Catros S, Fricain JC, Guillotin B, Pippenger B, Bareille R, Remy M, Lebraud E, Desbat B, Amédée J, Guillemot F, Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011, 3(2):025001. DOI: https://doi.org/10.1088/1758-5082/3/2/025001
[23] Devillard R, Pagès E, Correa MM, Kériquel V, Rémy M, Kalisky J, Ali M, Guillotin B, Guillemot F, Cell patterning by laser-assisted bioprinting. Methods Cell Biol 2014, 119:159-74. DOI: https://doi.org/10.1016/B978-0-12-416742-1.00009-3
[24] Koch L, Brandt O, Deiwick A, et al., 2017, Laser-assisted bioprinting at different wavelengths and pulse durations with a metal dynamic release layer: A parametric study. International Journal of Bioprinting, vol.3(1): 42–53.
[25] Keriquel, V., Oliveira, H., Rémy, M. et al., In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci Rep 2017, 7:1778. DOI: https://doi.org/10.1038/s41598-017-01914-x
[26] Ventura RD, An Overview of Laser-assisted Bioprinting (LAB) in Tissue Engineering Applications. Med Lasers 2021, 10:76~81. DOI: https://doi.org/10.25289/ML.2021.10.2.76
[27] Keating A, Mesenchymal stromal cells: new directions. Cell Stem Cell 2012, 10(6):709-716. DOI: https://doi.org/10.1016/j.stem.2012.05.015 Erratum in: Cell Stem Cell. 2012 Jul 6;11(1):136.
[28] Jones E, Yang X, Mesenchymal stem cells and bone regeneration: current status. Injury 2011, 42(6):562-8. DOI: https://doi.org/10.1016/j.injury.2011.03.030
[29] Simon-Yarza T, Bataille I, Letourneur D, Cardiovascular Bio-Engineering: Current State of the Art. J Cardiovasc Transl Res 2017, 10(2):180-193. DOI: https://doi.org/10.1007/s12265-017-9740-6
[30] Mathur A, Ma Z, Loskill P, Jeeawoody S, Healy KE, In vitro cardiac tissue models: Current status and future prospects. Adv Drug Deliv Rev 2016, 96:203-13. DOI: https://doi.org/10.1016/j.addr.2015.09.011
[31] Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014, 129(3):e28-e292. DOI: https://doi.org/10.1161/01.cir.0000441139.02102.80
[32] Gardin C, Ferroni L, Latremouille C, Chachques JC, Mitrečić D, Zavan B, Recent Applications of Three Dimensional Printing in Cardiovascular Medicine. Cells 2020, 9(3):742. DOI: https://doi.org/10.3390/cells9030742
[33] Cui H, Miao S, Esworthy T, Zhou X, Lee SJ, Liu C, Yu ZX, Fisher JP, Mohiuddin M, Zhang LG, 3D bioprinting for cardiovascular regeneration and pharmacology. Adv Drug Deliv Rev 2018, 132:252-269. DOI: https://doi.org/10.1016/j.addr.2018.07.014
[34] Gaebel R, Ma N, Liu J, et al., Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 2011, 32(35):9218-9230. DOI: https://doi.org/10.1016/j.biomaterials.2011.08.071
[35] Chua AW, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ, Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 2016, 4:3. DOI: https://doi.org/10.1186/s41038-016-0027-y
[36] Biedermann T, Boettcher-Haberzeth S, Reichmann E, Tissue engineering of skin for wound coverage. Eur J Pediatr Surg 2013, 23(5):375-82. DOI: https://doi.org/10.1055/s-0033-1352529
[37] Kellouche S, Martin C, Korb G, Rezzonico R, Bouard D, Benbunan M, Dubertret L, Soler C, Legrand C, Dosquet C, Tissue engineering for full-thickness burns: a dermal substitute from bench to bedside. Biochem Biophys Res Commun 2007, 363(3):472-8. DOI: https://doi.org/10.1016/j.bbrc.2007.08.155
[38] He P, Zhao J, Zhang J, Li B, Gou Z, Gou M, Li X, Bioprinting of skin constructs for wound healing. Burns Trauma 2018, 6:5. DOI: https://doi.org/10.1186/s41038-017-0104-x
[39] Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G, Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010, 363(2):147-55. DOI: https://doi.org/10.1056/NEJMoa0905955
[40] Pellegrini G, Lambiase A, Macaluso C, Pocobelli A, Deng S, Cavallini GM, Esteki R, Rama P, From discovery to approval of an advanced therapy medicinal product-containing stem cells, in the EU. Regen Med 2016, 11(4):407-20. DOI: https://doi.org/10.2217/rme-2015-0051
[41] Lynch AP, Ahearne M, Strategies for developing decellularized corneal scaffolds. Exp Eye Res 2013, 108:42-7. DOI: https://doi.org/10.1016/j.exer.2012.12.012
[42] Sorkio A, Koch L, Koivusalo L, Deiwick A, Miettinen S, Chichkov B, Skottman H, Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171:57-71. DOI: https://doi.org/10.1016/j.biomaterials.2018.04.034
Rights and Permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party materials in this article are included in the articles Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Creative Commons This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. You are not required to obtain permission to reuse this article. To request permission for a type of use not listed, please contact Biotechnology Kiosk.