
Genetic engineering of living cells is leading a new era of medicine. Using genetic engineering, scientists have programmed bacteria as a therapeutic delivery system to destroy tumors in mice (1). So far, designing a safe, efficacious, anti-tumor response without toxicity and within a solid tumor has
remained a challenge. However, recent research has demonstrated that bacteria could be programmed as an effective cancer therapy. In this editorial, we will describe recent advances made in genetic engineering of bacteria for cancer therapy.
Bacteria loaded with nanobodies:
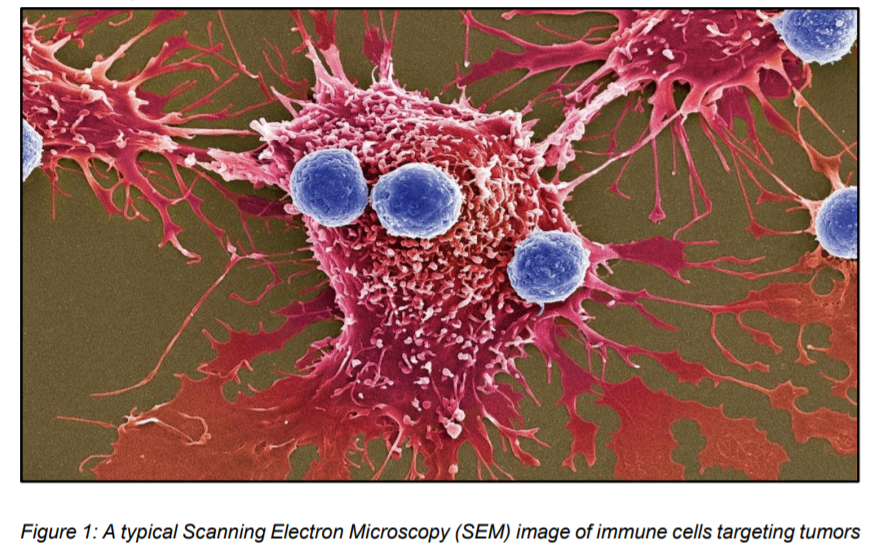
Our immune system is capable of targeting and killing cancer cells on its own (figure 1). However, certain tumor cells are resistant to immune cells and other macrophages due to a gene called CD47 (2). Normally, this gene encodes for a protein to coat the surface of Red Blood cells (RBCs) (3). This signal is interpreted as a kind of “Don’t eat me” signal and thus evades the check by immune cells. As RBCs age, CD47 proteins are lost and are
engulfed by immune systems, leading the way for new RBCs.
Mutations in cancer cells allow them to switch on the CD47 gene, rendering them to pass through immune cells and metastasize to other places, turning them to grow into tumors. A lot of efforts focused on targeting antibodies to attach to CD47 and mask the signal. However, due to large size of
antibodies, tunneling through large tumors becomes a daunting task (3). Also, injection into bloodstream could cause a random dispersion to other cells, causing side effects. In a recent paper published in Nature Medicine, scientists have engineered a nonpathogenic Escherichia coli strain capable of colonizing tumors and deliver immunotherapies within the tumor (1). Researchers programmed bacteria to turn off the ‘don’t eat me’ signal on tumors and triggering an anti-tumor response. This synergistic effect of E. coli capable of lysing within the tumor, inducing local inflammation, and blocking CD47 triggers proliferation of Tcells within the treated tumors for clearance. Further, these bacteria were programmed with the ability to produce smaller and potent antibodies against CD47, called nanobodies.
The researchers inserted a gene for nanobody turning them into nanobody factories, followed by an injection of 5 million of programmed bacteria into mice tumors (3).
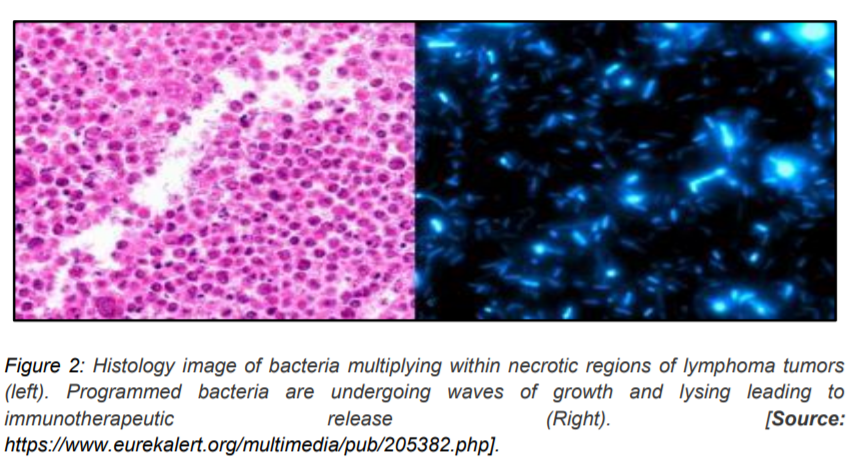
Additionally, the drug delivery system was set to mechanism of quorum sensing, which ensured a mass suicide for bacteria upon multiplication, releasing nanobodies (2). Moreover, the team also demonstrated that the fragments of dead bacteria could invade hidden cancer cells. These bacteria could multiply within the besieged tumor, commit suicide, deliver another round of nanobodies, and fragments (figure 2). The nanobodies owing to their small size, were easily cleared by the body, suggesting potential to reduce the side-effects of treatment. The team also demonstrated that this therapy could also shrink tumors at distant places, which could be due to an augmented recognition by immune cells. Further proof-of-concept tests, safety, and toxicology tests in various advanced solid tumor settings were shown in mouse models (4). The success of these tests could further translate to clinical trials in patients. The ultimate goal would be to treat some forms of metastatic cancer with pills of programmed bacteria. It remains to be seen how powerful these bacteria could be in a human setting.
This transformative approach could help prime a precise, efficient cancer therapy, without the side-effects of conventional drugs.
Genetically engineered bacteria:
Recently, multiple approaches have been developed to express reporter genes such as cytotoxic proteins, anticancer agents, and tumor-specific antigens (5). Clostridia strains including (C acetobutylicum and C beijerinckii) have demonstrated immense promise to express genes encoding bacterial enzymes (cytosine deaminase, nitro reductase), or tumor necrosis factor (TNF-α) to trigger antitumor effects. Clinical trials with programmed S typhimurium and Clostridium novyi-NT expressing HlyE or Stx2 or rec A (crucial protein for DNA repair and maintenance) have demonstrated their ability to activate the host cytokines including Interleukin-2 (IL-2), IL-4, IL-8, CC chemokine 21, leading to effective tumor reduction (6)
Bacteria as immunotherapeutic agents:
Cancer immunotherapy usually involves targeting specific immune responses within the host system to target cancer cells (5). Bacterial infections caused by C novyi could lead to the release of heat shock proteins (Hsp70), and pathogen-associated molecular patterns (PAMPs). While Hsp70 drives dendritic cell maturation, PAMPs activate interferon gamma (IFN-γ) and Th1dependant cell-mediated response, driven
by CD8+ cells (5). Research shows that CD8+ cells derived from C novyi NT-treated mice stimulates acquired immunity in a tumor-specific model. In another interesting approach, scientists took advantage of a type-3 secretion system (T3SS) of S typhimurium to infect tumor cells. However, more research is underway to fully understand its mechanism of action. Similarly, some bacterial compounds including CpG oligonucleotides could be used for dendritic cell stimulation and complete regression of B16F10 melanoma tumors (5).
Bacterial toxins or enzymes as anticancer agents:
Various bacterial toxins have the capability to suppress the immune response of the infected host. Researchers are investigating the potential of these toxins as an anti-cancer therapy, as they are potent inhibitors of antibodies and cytokines (7). Among these toxins are highly specific enzymes with the ability to alter their substrates in the cytosol, change cellular function, morphology, and even kill the host cells. Cytolysin A (Cly A), is a bacterial toxin that induces caspasemediated cell-death by making pores in eukaryotic cell membranes (5). Studies have demonstrated that by treating mice with S typhimurium or E. coli strains expressing the Cly A toxin inhibited tumor growth. Similarly,
TNF-related apoptosis-inducing ligands (TDAI-I), FAS ligand (FAS -I), and TNFA selectively lead to programmed cell death via death receptor pathways. Zheng et al developed a biotic/abiotic hybrid system, in which they combined carbon nitride (C3N4) with an E coli strain to produce nitric oxide (NO) (8). C3N4 loaded bacteria were accumulated within the tumor, with an enhanced ability (80% inhibition of tumor growth) to destroy cancer cells. Cyclomodulins, a class of bacterial toxins, that could inhibit or activate the eukaryotic
cell cycles are also being intensely researched upon as anti-cancer agent including CNFs and CDTs. These toxins could be associated with reduced side effects compared to conventional anti-tumor activity (5).
Conclusions
Despite a lot of progress in the field, there are still many challenges for widespread use of programmable bacteria as anti-cancer agents. Bacterial toxicity, DNA instability, nlimited targeting efficiency, choice of safe bacterial strains, and testing combination with other therapies are some of the current obstacles. However, with the intense research efforts, further advances in synthetic biology combined with effective
genetic engineering, unique biochemical properties of bacteria could be developed for a whole host of advanced applications for treating cancer.
References
1. Chowdhury S, Castro S, Coker C, Hinchcliffe TE, Apaia N, and Danino T. July (2019) Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 25(7): 1057- 63.
2. Paddock C. July (2019) Programming bacteria to fight cancer. Medical News Today.
3. Zimmer C. July (2019) New Weapons Against Cancer: Millions of Bacteria Programmed to Kill. The New York Times.
4. Bacteria engineered as Trojan horse for cancer immunotherapy. July (2019) ScienceDaily.
5. Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M, Lohrasbi V, Mohammadzadeh N, Amiriani T, Krutova M, Amini A, and Kouhsari E. June (2019). Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer
Med. 8(6):3167-3181.
6. Nallar SC, Xu D‐Q, Kalvakolanu DV. (2017) Bacteria and genetically modified bacteria as cancer therapeutics: Current advances and challenges. Cytokine. 89:160‐172.
7. Zahaf N‐I, Schmidt G. (2017) Bacterial toxins for cancer therapy. Toxins. 9:236.
8. Zheng D‐W, Chen Y, Li Z‐H, et al. (2018) Optically controlled bacterial metabolite for cancer therapy. Nat Commun;9.