
Abstract
Corona Virus Disease 2019 (COVID-19) has been designated by the World Health Organization as a life-threatening global pandemic caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2). The existing diagnostic practices are mainly based on real-time fluorescent PCR (RT-PCR), which is considered the clinical standard for SARS-CoV-2 nucleic acid detection. The ongoing efforts are focused on to develop new rapid diagnostics including point-of-care diagnostics and surveillance technologies to detect SARS-CoV-2 early in order to control the disease and mitigate it in a timely fashion. Here, we present an overview of the current state-ofthe-art of diagnostics of SARS-CoV-2 and the limitations of the existing technologies. We describe the emerging new promising diagnostic technologies that overcome the limitations by offering rapid, point-of-care diagnostic abilities for patients. Finally, we discuss innovative concepts and future directions in developing capabilities on smart surveillance technologies for mitigating the current challenges and also to prevent future global pandemic such as COVID19.
*E-Mail: megha@biotechkiosk.com
Existing Diagnostic Practices for SARSCoV-2 Pandemic
The novel severe acute respiratory syndrome coronavirus (SARS-CoV-2, COVID-19) was first detected in December 2019 in the Hubei Province of China. Since the outbreak of this highly infectious disease, the COVID-19 pandemic has brought devastating and lifealtering effects across the globe by infecting hundreds of thousands of people, which resulted in tens of thousands of deaths in as many as 182 countries so far [1]. During these difficult times caused by COVID-19 pandemic that has seen a continued spread of the disease, it is critically important that we have effective diagnostic tools and practices in place that can play a key role in the containment of COVID-19. Effective diagnostics can enable the rapid implementation of control measures that can limit the spread through infected patient identification and subsequent isolation. This also enables contact tracing including identifying people that may have come in contact with an infected patient. Figure 1 describes a workflow for the existing diagnostic practices for COVID-19 [2].
Regarding the employed diagnostics of this disease, the computed tomography (CT) images that were obtained earlier during the time of disease breakout suggested pneumonia-like symptoms and abnormal lung in infected people [3]. The negative stained transmission electron microscopy (TEM) was used to identify the morphology of the virus and the images revealed virus with diameter ranging from 60 to 140 nm that comprised an envelope with protein spikes, and genetic material [3, 4].
In addition to CT based diagnostics and TEM based morphological images, a multiplex polymerase chain reaction (PCR) panel of known pathogens was employed to analyze the samples from infected patients. This helped identification of the previously unknown pathogen as an RNA virus through next generation sequencing [3]. The virus was later named SARS-CoV-2 because its genome sequence showed similarity with SARS-CoV, the virus that caused SARS in 2002−2003. Researchers were able to use the whole genome sequence to develop PCR kits to diagnose patients suffering with COVID-19 [3, 4]. PCR/RT-PCR, especially, real-time (RT) PCR/RT-PCR has been employed as a highly sensitive and specific method for detection of infectious diseases such as COVID-19. To this end, PCR/RTPCR-based methods have been employed to detect nucleic acid (e.g., RNA) of novel coronavirus SARS-CoV-2 for early diagnosis of COVID-19 [2].
RT-PCR is currently used for the diagnosis of COVID-19 along with screening with CT scans. However, PCR/RT-PCRbased methods have limitations due to the fact that they are typically restricted in a centralized clinical laboratory that requires sophisticated equipment and well-trained personnel. Further, the less availability of enough PCR reagent kits is another issue that has been faced to keep up with the high demand. In addition, another challenge is the reliance of RT-PCR on the presence of detectable SARS-CoV-2 in the sample collected [2]. Because of this limitation, it is difficult to identify infection of SARS-CoV-2 in a recovered asymptomatic patient who was infected with the disease before. So, this does not allow to enforce control measures. On the other hand, CT based imaging procedures are quite expensive and require technical expertise that also cannot specifically diagnose COVID-19 [2]. Thus, these methods are not considered suitable for simple, rapid, point-of-care diagnostic applications for SARS-CoV-2 [2, 5].

Figure 1: A typical workflow showing the steps of diagnostic practices for COVID-19 [Source: ACS Nano (2020)].
Need for New Diagnostic and Surveillance Strategies
It is of great concern worldwide that COVID19 might persist well beyond 2020. This places a significant stress on the global healthcare systems. In view of this, it is believed that new innovations in research to develop next generation diagnostics and surveillance technologies are needed to combat the disease. The World Health Organization (WHO) has therefore, recommended for an immediate priority for COVID-19 diagnostics research for the development of point-of-care nucleic acid and protein tests and detection. Point-of-care tests are advantageous as they are costeffective, handheld devices that can be leveraged to diagnose patients outside of centralized facilities including community centers that can reduce the burden on clinical laboratories. The goal is to integrate these tests into multiplex panels in future [2-4].
As for improving efforts on surveillance technologies, in addition to nucleic acid based tests, serological tests using proteins are being considered. These tests are considered superior than nucleic acid tests taken alone due to the reason that they have the benefits of detection after recovery. This can be leveraged to help clinicians to track both sick and recovered patients, which can provide a better statistics of total SARS-CoV-2 infections [2]. Therefore, it is of immense importance from a healthcare point of view to develop novel diagnostic technologies enabling rapid and simple identification of people infected with SARS-CoV-2. This could potentially enable appropriate isolation measures and contact tracing that would be helpful to reduce the transmission of SARS-CoV-2 [2]
Rapid Diagnostic Technologies: Looking Beyond the Current State-of-the-Art
COVID-19 pandemic has created a global healthcare crisis that has called for urgent development of point-of-care tests and multiplex assays beyond the available technologies. To this end, the identification and sequencing of SARS-CoV-2 has enabled current research and developments to focus on nucleic acids and viral proteins, antigens and antibody based rapid diagnostics. These diagnostics are supposed to act as a first line of defense against an outbreak of a disease. More advanced diagnostics are believed to include serological tests (i.e., blood tests for specific antibodies) based technologies. These technologies are thought to be easier to administer that may complement nucleic acid tests for diagnosing COVID-19 infection [2]. Researchers are paying a lot of attention to nucleic acid tests using isothermal amplification that are currently in development for SARS-CoV-2 detection [2]. Isothermal amplification techniques include recombinase polymerase amplification, helicase-dependent amplification, and loopmediated isothermal amplification (LAMP). They can be conducted at a single temperature without requiring specialized laboratory equipment that can provide high analytical sensitivities to PCR [6]. RT-LAMP employs DNA polymerase with four to six primers to bind to six distinct regions on the target genome [2]. Researchers have developed and clinically tested reverse transcription LAMP (RT-LAMP) tests for SARS-CoV-2 [7]
Current research has also focused on viral protein antigens and antibodies that can be used for diagnosing COVID-19 [8]. Among currently considered point-of-care approaches in diagnostics for COVID-19, lateral flow antigen based point-of-care detection for SARS-CoV-2 is under development for diagnosing COVID-19 [9]. Another point-of-care approach based on microfluidic devices is being considered. Microfluidic devices are essentially based on palm-sized chip etched with micrometersized channels and reaction chambers [2]. The function of chip is to mix and separate liquid samples using electro kinetic, capillary, vacuum, and/or other forces. Microfluidics based diagnostics for SARS-CoV-2 offer a number of advantages that include miniaturization, small sample volume, rapid detection times, and portability. All these point-of-care diagnostic technologies are envisioned to be adapted to detect SARSCoV-2 RNA or proteins [2].
CRISPR-Cas12a Assay for Rapid, Ultrasensitive and Visual Detection of SARS-CoV-2
RNA-guided CRISPR/Cas nucleasebased nucleic acid detection has been shown very promising for next-generation molecular diagnostics technology. This is due to the reason for its high sensitivity, specificity and reliability [5]. To this end, important Cas nucleases (e.g., Cas12a, Cas12b and Cas13a) have been shown by the researchers to perform strong collateral cleavage activities. These include a crRNAtarget-binding activated Cas that can indiscriminately cleave surrounding nontarget single-stranded nucleic acids [5].
Researchers employed CRISPRCas12a (termed “AIOD-CRISPR”) assay for rapid, ultrasensitive, specific and visual detection of nucleic acid for visual real time SARS-CoV-2 diagnostics (Figure 2) [5]. Dual crRNAs were introduced to initiate highly efficient CRISPR-based nucleic acid detection. In their AIOD-CRISPR assay, researchers all components for nucleic acid amplification and CRISPR detection that were mixed in a single, one-pot reaction system and incubated at a single temperature. This eliminated the requirement for separate pre-amplification and amplified product transferring. Researchers then engineered the AIOD-CRISPR assay to detect severe SARS-CoV-2 (Figure 2) [5]. The advantage of the test results of the AIOD-CRISPR assay was the direct visualization by the naked eye. Therefore, this diagnostic technology was envisioned by the researchers as CRISPR-based nextgeneration molecular diagnostics towards point-of-care applications for COVID-19 [5].
Figure 2A shows a pUCIDT-AMP plasmid containing 384 nt SARS-CoV-2 N gene cDNA (N plasmid) that was first prepared as the target to develop the AIODCRISPR assay. Researchers demonstrated that AIOD-CRISPR assay could detect 1.3 copies of SARS-CoV-2 N plasmids in both real-time and visual detections within 40 min. This offers a rapid and nearly single-molecule level sensitive detection (Figure 2B) [5]. The reaction with SARS-CoV-2_PC showed the positive signal in both real-time and visual detections, which demonstrated the high specificity without cross reactions for nonSARS-CoV-2 targets by the developed AIOD-CRISPR assay (Figure 2C) [5]
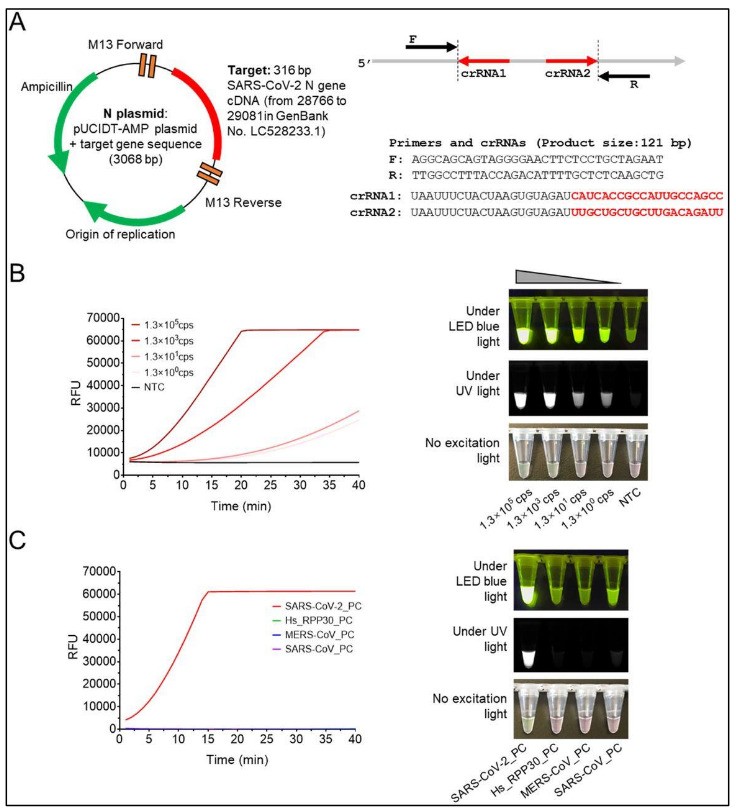
Figure 2: The AIOD-CRISPR assay for SARS-CoV-2 N DNA detection. (A) The pUCIDT-AMP plasmid is shown that contains 316 bp SARS-CoV-2 N gene cDNA (N plasmid) and the primers and crRNAs. (B) Real-time AIOD-CRISPR detection is shown with various copies of SARS-CoV2 N DNA. (C) Specificity assay of the AIOD-CRISPR assay on SARS-CoV-2 N detection is shown [Source: bioRxiv (2020)].
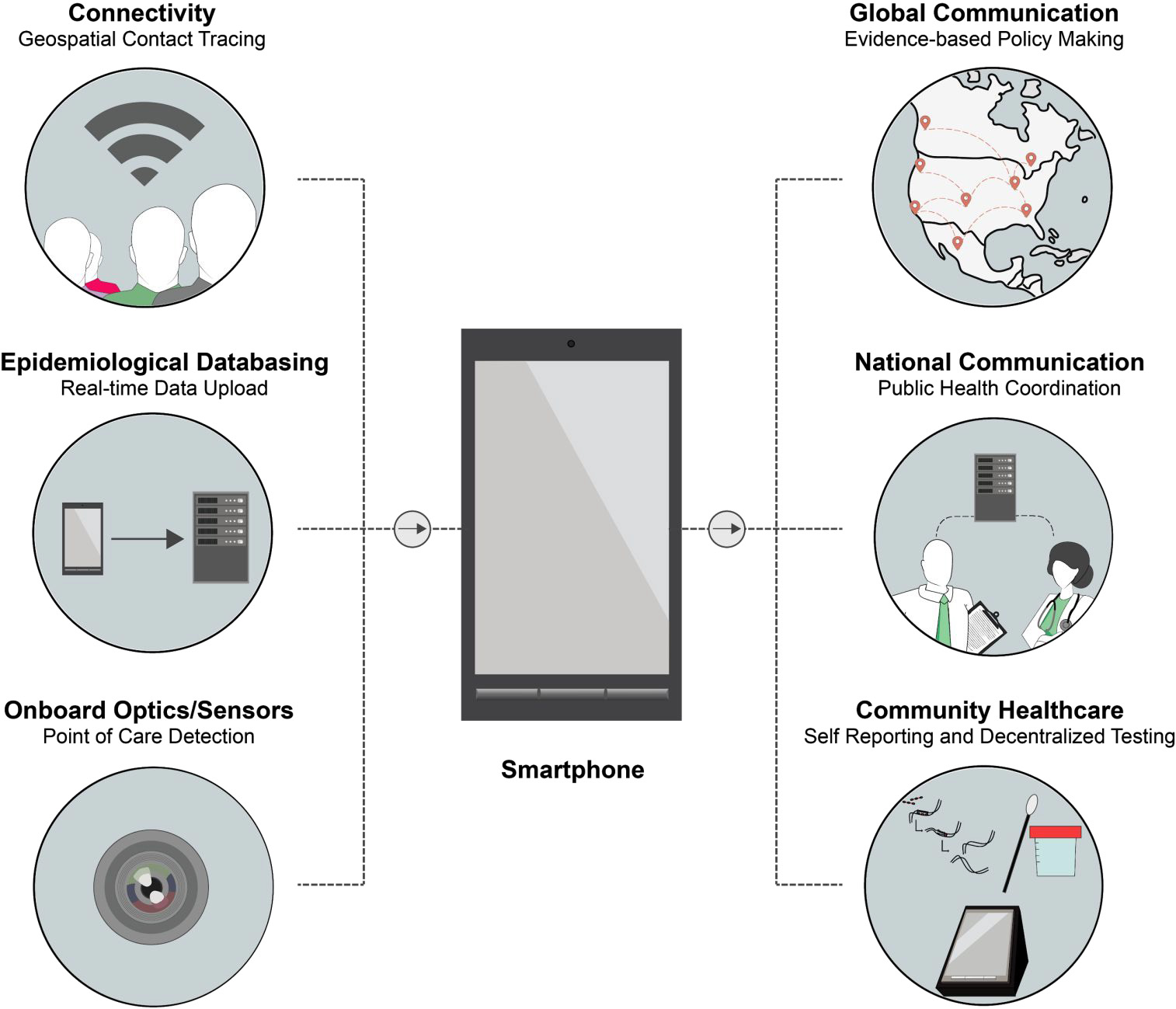
Figure 3: Schematic presentation showing the envisioned role of smartphones in diagnostics for COVID-19 [Source: ACS Nano (2020)].
Innovative Mass Surveillance and Monitoring Technology to Prevent Future Outbreaks
Studies have strongly suggested that insufficient communication and underreporting have been important factors that have contributed to some extent the global spread of COVID-19 [10]. It is believed that a robust system of networks consisting of mass surveillance with rapid diagnostics can help public health officials monitor virus spread and proactively identify areas with increasing infections. Such a system in place can also be leveraged to anticipate surge capacity needs, and deploy needed resources to the appropriate areas accordingly. However, the functioning of such a system will be impaired in absence of clear and transparent collaboration and communications between nations at the international level, and between federal and state/principal public health laboratories, hospitals, government agencies, and communities at the domestic level [2].
Researchers are considering to employ the innovative combination of diagnostics and smartphones that is believed to provide greater communication and surveillance (Figure 3) [2]. Smartphones are considered attractive choice because they possess the connectivity, computational power, and hardware that can be facilitated for electronic reporting, epidemiological data basing, and point-of-care testing [11]. It is believed that smartphones can be made a widely accessible technology to coordinate responses that include real-time geospatial information empowering national and global health agencies to implement coordinated control strategies during large outbreaks like COVID-19 [11].
Concluding Remarks
Rapid diagnostics, surveillance and monitoring are critically important components in medical practices to deal with COVID-19 pandemic. A robust containment and mitigation system including smart, accurate and rapid diagnostics would curb the spread of highly infectious SARS-CoV-2 disease, while providing healthcare workers the necessary resources that protect the frontline workers fighting the pandemic.
References for further reading
1. World Health Organization: Coronavirus Disease (COVID-19) Pandemic, https://www.who.int/emergencies/diseas es/novelcoronavirus-2019.
2. Buddhisha Udugama, Pranav Kadhiresan, Hannah N. Kozlowski, Ayden Malekjahani, Matthew Osborne, Vanessa Y.C. Li, Hongmin Chen, Samira Mubareka, Jonathan Gubbay, and Warren C.W. Chan, Diagnosing COVID19: The Disease and Tools for Detection, ACS Nano, (2020), DOI: https://doi.org/10.1021/acsnano.0c02624
3. Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K. S. M.; Lau, E. H. Y.; Wong, J. Y.; Xing, X.; Xiang, N.; Wu, Y.; Li, C.; Chen, Q.; Li, D.; Liu, T.; Zhao, J.; Liu, M.; Tu, W.; Chen, C. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus Infected Pneumonia. N. Engl. J. Med. (2020), DOI:10.1056/NEJMoa2001316
4. Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. (2020), 382 (8), 727–733, DOI: 10.1056/NEJMoa2001017
5.Xiong Ding, Kun Yin, Ziyue Li, Changchun Liu, All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV virus, bioRxiv (2020), doi: https://doi.org/10.1101/2020.03.19.99872 4
6. Craw, P.; Balachandran, W, Isothermal Nucleic Acid Amplification Technologies for Point-of-Care Diagnostics: A Critical Review, Lab Chip, 12, 2469 (2012), DOI:10.1039/c2lc40100b
7. Yang, W.; Dang, X. et. al., Rapid Detection of SARS-CoV-2 Using Reverse Transcription RT-LAMP Method., medRxiv, March 3, (2020), DOI: 10.1101/2020.03.02.20030130
8. To, K. K.-W.; Tsang, O. T.-Y.; Leung, W.- S.; Tam, A. R.; Wu, T.-C.; Lung, D. C.; Yip, C. C.-Y.; Cai, J.-P.; Chan, J. M.- C.; Chik, T. S.-H., Temporal Profiles of Biotechnology Kiosk, 2, 4 (2020) ISSN 2689-0852 Page 22 Viral Load in Posterior Oropharyngeal Saliva Samples and Serum Antibody Responses during Infection by SARSCoV-2: An Observational Cohort Study. Lancet Infect. Dis. (2020), DOI: 10.1016/S1473- 3099(20)30196-1
9. Xiang, J.; Yan, M.; Evaluation of EnzymeLinked Immunoassay and Colloidal GoldImmunochromatographic Assay Kit for Detection of Novel Coronavirus (SARSCov-2) Causing an Outbreak of Pneumonia (COVID-19), medRxiv, March 1,(2020), DOI: 10.1101/2020.02.27.2002 8787
10. Sun, H.; Dickens, B. L. et. al., Estimating Number of Global Importations of COVID19 from Wuhan, Risk of Transmission Outside Mainland China and COVID-19 Introduction Index between Countries Outside Mainland China, medRxiv, February 20, (2020), DOI: 10.1101/2020.02.17.200240 75
11. Wood, C. S.; Thomas, M. R.; Budd, J.; Mashamba-Thompson, T. P.; Herbst, K.; Pillay, D.; Peeling, R. W.; Johnson, A. M.; McKendry, R. A.; Stevens, M. M. Taking Connected Mobile-Health Diagnostics of Infectious Diseases to the Field, Nature , 566 (7745), 467– 474, (2019), https://doi.org/10.1038/s41586-019- 0956-2