
Neurodegenerative disorder detection based on Bodily Fluids
Examination of CSF biomarkers for their use in various neurodegenerative disorders is an exciting area of research. The clinical use of CSF biomarkers is currently most relevant to the diagnosis of Alzheimer’s disease (AD). For AD, Classical markers are T-tau, P-tau and Aβ42. Published evidence demonstrates CSF Aβ42, CSF T-tau and CSF P-tau could be used as screening or diagnostic tools for AD [1] (Figure 1). Elevated levels of CSF Aβ42, and lowered CSF T-tau and CSF P-tau characterize AD. Recent studies show CSF Aβ42/Aβ40 biomarker ratio to be superior to individual constituents for diagnosis of AD with greater sensitivity, specificity, and accuracy [1]. Similarly, CSF T-tau/ Aβ42 ratio could be helpful to predict progression from preclinical to clinical AD. Studies also suggest that different forms of P-tau could have a unique role in identifying the pathology of AD [2]. However, further work is required to analyze the profiles of these CSF biomarkers in patients with AD. In another interesting breakthrough, scientists have identified biomarkers in CSF that are nonspecific to AD pathology. These biomarkers could signify further improvement in the efficacy and specificity for clinical applications. Some examples include YKL-40, NFL, NSF, markers of neuronal damage and neuronal injury respectively [1, 3]. Intense ongoing efforts are on to identify other potential CSF biomarkers including vascular growth factors, CSF/serum albumin ratios, however further research is required.
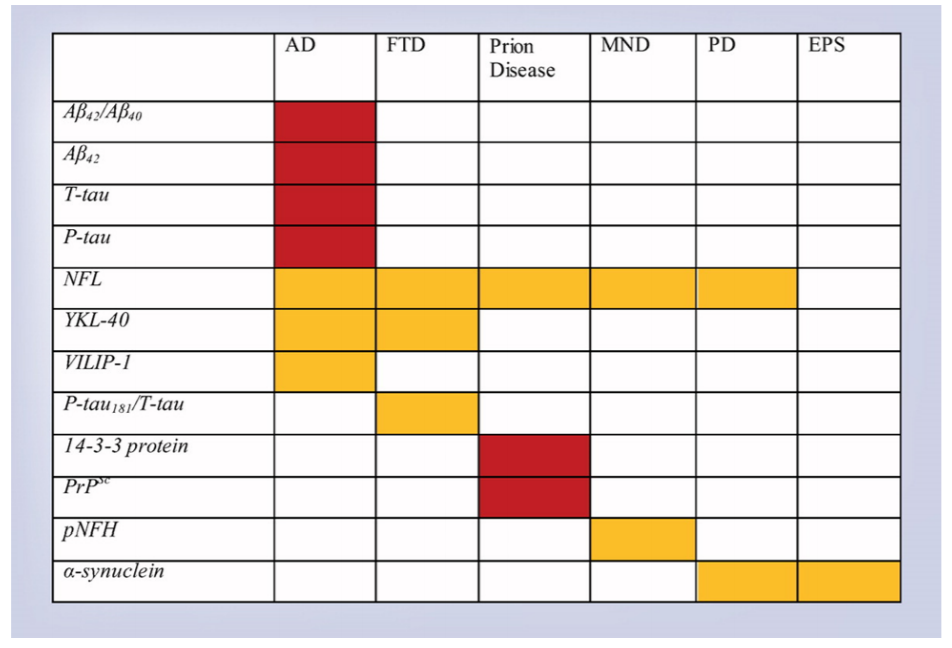
Figure 1. Cerebrospinal fluid biomarkers in neurodegenerative disorders. Red = useful. Gold = promising, requires further study [Source: Future Neurol, 14 (1) 2019].
It has been tough to find a specific biomarker for Frontotemporal dementia (FTD) due to its multiple clinical features and pathological subtypes. CSF P-tau/Ttau ratio has shown promising results as a useful biomarker for FTD [4]. Studies also suggest that a combination of YKL40 and NFL could act as valuable CSF biomarkers [1]. Additional studies are required to further assess the applicability of these markers.Researchers have developed a new technique called read time quakinginduced conversion (RT-QUIC), which is able to detect the presence of pathological prion proteins in CSF [5, 6]. This assay has revealed successful results in the diagnosis of sporadic prion disease, a fatal neurodegenerative disease caused by the formation and propagation of misfolded proteins. Ongoing research is focused on improving the clinical utility of the traditional CSF marker 14-3-3 by combining with other biomarkers [1]. Again, additional studies are required to understand the clinical application.
CSF biomarkers have also been identified for motor neuron disease. The most promising results have been found with NFL, pNFH, lipids, and the Sonic Hedgehog protein [7, 8]. Studies have also met with some success in identifying some CSF biomarkers for Parkinson’s disease (PD) and other extrapyramidal syndromes [1].
Taken together, more research is required to identify the proteome profile from patients for different combinations or ratios of CSF biomarkers for detecting neurodegenerative diseases. Thorough understanding of CSF biomarkers could help in developing personalized neurology for patients and aid in the clinical applicability in neurodegenerative diseases.
2. Blood
Over the past few years, efforts are on to identify novel blood markers to detect the underlying pathologies of neurodegenerative diseases, including AD, PD, and chronic traumatic encephalopathy (CTE). Blood-based biomarkers would mean less invasive testing, earlier diagnosis, quick and effective treatment [9]. With the advent of recent technologies, including Quanterix’s Simoa (single-molecule array), detection of neurological markers in the blood has improved significantly [10]. Quanterix is able to screen patient samples with 72000 microscopic beads coated with a capture antibody specific for the biomarker of interest. The complex is then detected with a fluorescent marker with a high signal-to noise ratio and increased sensitivity. Quanterix has successfully measured neurofilament (NFL), a biomarker for neuronal damage in blood for concussion, CTE and AD [11]. Another technology, Olink’s Proximity Extension Assay (PEA), based on antibody probes linked to DNA tags, can detect about 1000 biomarkers in less than one drop of blood.
Recently, researchers using high throughput multiplexed Xmap Luminex assay reported a set of eight plasma proteins (BDNF, AGT, IGFBP-2, OPN,
cathepsin D, SAP, complement C4, and TTR) for AD diagnosis with high sensitivity and specificity [12]. Some of these proteins are implicated in the
pathology of AD for the formation of Aβ fibrils, cell proliferation, and death in previous studies as well. Some other recent studies have identified biomolecules such as creatine, 5- hydroxycytosine, serine, phospholipids, myo-inositol, glutamate, N-acetylaspartate, blood dehydroepiandrosterone, vary with the progression of AD [13]. Further research is required to validate the results in a large independent cohort. Moreover, researchers have also determined elevated levels of the P-tauproteins within neuron-derived exosomes (NDEs), isolated from plasma Exosomes are released from neurons and represent a subtype of membrane vesicle for removal of excess proteins, shuttle cargo between cells. Various biomarkers measurable in NDEs including P-tau protein could accurately predict AD progression.
In another breakthrough research, scientists identified RNA blood biomarkers in several independent cohorts to rule out a high rate of misdiagnosis rate between PD and atypical parkinsonian disorders (APD) [15]. The team evaluated the diagnostic potential of nine previously identified RNA biomarkers in 138 blood samples from PD, PSP patients, and healthy controls. The success of these results has encouraged scientists to evaluate the results in a larger cohort and advance the biomarkers into the clinic. With intense ongoing research, detection of neurodegenerative diseases using noninvasive blood-based biomarkers will be useful.
3. Urine
Compared to other body fluids, urine remained mostly neglected for detecting neurodegenerative diseases. However, recent research provides evidence that urine is a promising candidate for effective biomarkers for diagnosis, and monitoring of various neurodegenerative diseases including AD, PD, multiple sclerosis (MScl, and transmissible spongiform encephalopathies (TSEs) [16]. Two recent studies in transgenic mice identified potential biomarkers for AD using different methods. Fukuhara et al analyzed the urinary metabolome of Tg2576 model of transgenic AD mice using NMR and identified 3- Hydroxykynurenine, homogentisate, and tyrosine as biomarkers of AD before the onset of dementia [17]. Moreover, 1- methylnicotinamide, 2-oxoglutarate, citrate, urea, dimethylamine, trigonelline, and trimethylamine were characterized as effective biomarkers of late-stage AD. Another study using liquid chromatography-MS (LC-MS) identified methionine, 5-hydroxyindoleacetic acid, desaminotyrosine, taurine, and N1- acetylspermidine were identified as promising biomarkers in the TgCRND8 model of transgenic mice [18].
Studies indicate that differences exist between the levels of several urinary metabolites between PD patients and healthy control human subjects. These metabolites are associated with fatty acid beta-oxidation, metabolism of phenylalanine, histidine, tyrosine, nucleotide, and tryptophan. The biomarkers include as hydroxylauroylcarnitine, phenylacetic acid, histidine, dihydrocortisol, and acetylserotonin [16]. Similarly, some studies have reported the alterations in urinary proteins in MScl patients versus healthy controls. The disease-related proteins may reflect abnormalities in the central nervous system and could indicate pathological changes in the brain.
Researchers have also identified promising biomarkers using samples from patients or animal models of TSEs, a group of nervous system disorder
associated with the aggregation of prion protein (PrPd). Moreover, preliminary observations report elevated levels of common neurotrophic receptor (p75) in urine samples of ALS patients compared to healthy individuals [19]. The studies indicate that urinary p75 could act as an effective prognostic biomarker of motor neuron degeneration for ALS. Taken together, urinary signatures might open doors to alternative approaches in the diagnosis of various neurodegenerative disorders.
4. Saliva Concussion diagnosis
It turns out that biomarkers in the saliva could facilitate early diagnosis of a multitude of neurological diseases esp the cases of concussion or neuronal injury [20]. Traditional approaches to detecting neuronal damage rely on the identification of proteins in the blood. However, limited diffusion of these proteins across the blood-brain-barrier leads to a difficult diagnosis of concussion [21]. Therefore, smaller molecules specific to the neuronal injury response, that could easily cross the blood-brain barrier and capable of an easy measurement could provide valuable information to diagnose the disease.
Researchers at the Penn State College of Medicine have identified miRNAs, as ideal candidates for detecting and characterizing concussions [22]. miRNAs are abundant short, non-coding mRNAs that affect the expression of genes in response to different conditions including disease or injury [23]. The researchers predict that the miRNAs might be able to predict the presence and duration of concussions. They collected and examined the saliva from 52 concussion patients for the analysis of the miRNA expression. The team isolated five miRNAs from the patients with the prolonged symptoms of the concussion. Salivary miRNA represents an objective biomarker for diagnosis and management of concussion. Additional
follow up studies with larger cohorts have yielded promising results and could be a valuable future point-of-care concussion tool.
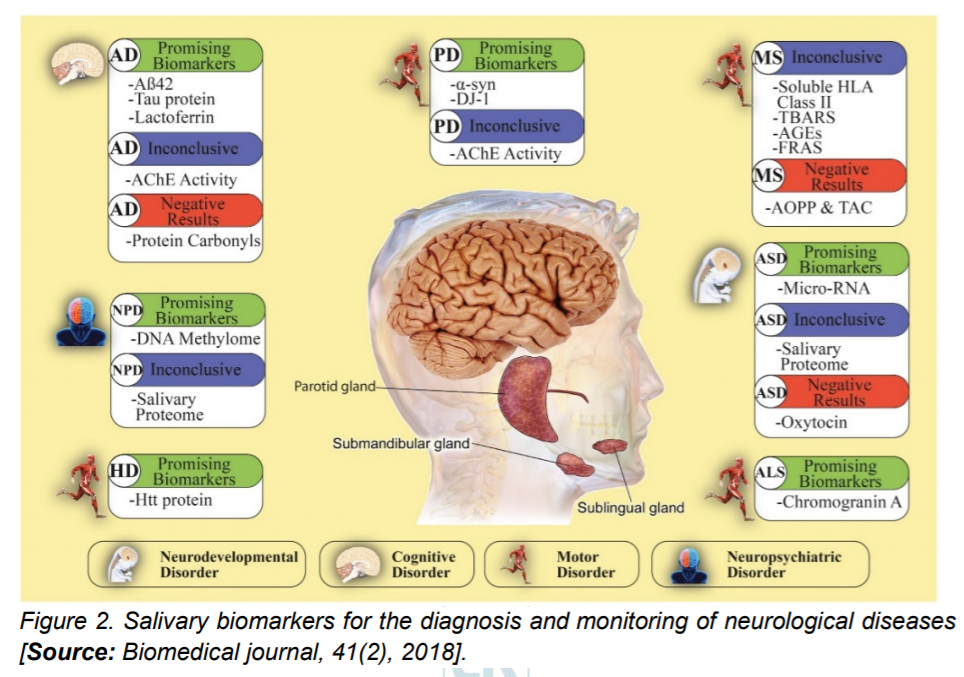
Researchers have also detected several proteins implicated in the pathological development of neurological diseases in saliva (Figure 2). For example, a toxic oligomeric form of salivary alphaSynuclein (α-syn) is higher in patients with PD than in healthy controls. Similarly, both Huntington and amyloid beta peptides, proteins implicated in Huntington’s and Alzheimer’s disease are capable of being detected in the saliva and could allow easy detection of these diseases [24]. Additional studies are required to verify and implement these early studies for a sensitive, specific, high-throughput, low-cost and portable salivary diagnostics.
Screening for AD, Parkinson’s disease may become a part of the future eyeexam protocols as a means to improve our understanding and detection of these two debilitating diseases. Research indicates that tear fluid could lead to the characterization of neurodegenerative diseases [25]. Tear fluid, a part of the innate immune system, creates a chemical barrier at the surface of the eye and secrets various antibacterial and immunomodulatory proteins for inhibiting bacterial growth. Changes in the retinal morphology and blood flow in AD could alter the microenvironment of the eye leading to the alterations in the tear proteins (Figure 3).
 Alteration in the tear flow rate, total tear protein concentration, and changes in the chemical barrier composition of tears specific to AD have been demonstrated [26]. Combination of 4 tear proteins lipocalin-1, dermacidin, lysozyme C, and lactritin exhibited 81% sensitivity and 77% specificity for AD diagnosis. Several miRNAs, namely hsa-miR-106, -153, -101, -29, -107 are implicated in regulating amyloid production and have been isolated in tear fluid [26]. Therefore, these miRNAs could have a profound influence on the early diagnosis of AD. In another research, a significant increase in the levels of totaltau and Aβ42 were observed in a cohort of 25 patients with AD. However, studies with larger sample sizes are required to confirm the results.
Alteration in the tear flow rate, total tear protein concentration, and changes in the chemical barrier composition of tears specific to AD have been demonstrated [26]. Combination of 4 tear proteins lipocalin-1, dermacidin, lysozyme C, and lactritin exhibited 81% sensitivity and 77% specificity for AD diagnosis. Several miRNAs, namely hsa-miR-106, -153, -101, -29, -107 are implicated in regulating amyloid production and have been isolated in tear fluid [26]. Therefore, these miRNAs could have a profound influence on the early diagnosis of AD. In another research, a significant increase in the levels of totaltau and Aβ42 were observed in a cohort of 25 patients with AD. However, studies with larger sample sizes are required to confirm the results.Tear proteins could also facilitate the diagnosis of PD at different stages of the disease. Researchers from the University of Southern California identified an altered protein composition of basal and reflex tears in patients with PD versus healthy controls. They show that the tear levels of alpha-synuclein may act as a biomarker for PD diagnosis. Moreover, increased TNF-α levels were reported in tears of patients with PD [26].Conclusion:Neurodegenerative diseases are hard to diagnose due to significant clinical overlap and lack of clinical symptoms. However, scientists have made
breakthroughs in identifying and isolating several biomarkers from bodily fluids to achieve an early diagnosis to develop a personalized treatment to the patients. With the current advances, investigating biomarkers for neurodegeneration in the bodily fluids would bring us one step closer to find more disease biomarkers and potentially improve the quality of life for patients.
Dr. Navneeta Kaul holds a doctorate degree in biology from the University of Denver in August 2018. She works as a scientific consultant in the Biotech industry and is involved in regulatory and clinical affairs consulting. As a researcher, she has experience in biochemical and molecular biology techniques like cloning, PCR, real-time PCR, western blotting, immunoprecipitation, chromatogram analysis, live cell, and fixed cell imaging. She is passionate about communicating new technologies and research advances to a diverse audience. Dr. Kaul can be reached at navneetakaul@gmail.com
1. Robey & Panegyres. (2019). Cerebrospinal fluid biomarkers in neurodegenerative disorders. Future Neurol,, 14 (1).
2. Kidermet-Piskac S, Babic Leko M, Blazekovic A et al. (2018). Evaluation of cerebrospinal fluid phosphorylated tau231 as a biomarker in the differential diagnosis of Alzheimer’s disease and vascular dementia. CNS Neurosci. Ther., 24(8), 734–740.
3. Haque A, Polycyn R, Matzelle D, Bainik NL. (2018). New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration and neuroprotection. Brain Sci., 8(2), 33.
4. Borroni B, Benussi A, Archetti S et al. (2015). Csf p-tau181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph. Lateral Scler. Front. Degener. 16(1-2), 86–91.5. Atarashi R, Sano K, Satoh K, Nishida N. (2011. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion, 5(3), 150– 153.6. Kang HE, Mo Y, Abd Rahim R, Lee HM, Ryou C. 2017. Prion diagnosis: application of real-time quakinginduced conversion. Biomed. Res. Int, (1), 1–8.7. Li D, Shen D, Tai H, Cui L. (2016). Neurofilaments in CSF as diagnostic biomarkers in motor neuron disease: a meta-analysis. Front. Aging Neurosci., 8, 290.8. Oberstadt M, Classen J, Arendt T, Holzer M. (2018). TDP-43 and cytoskeletal proteins in ALS. Mol. Neurobiol., 55(4), 3143–3151.
9. Henrik Zetterberg. (2019). Bloodbased biomarkers for Alzheimer’s disease-An update. Journal of Neuroscience methods, 319, 2-6.
10. https://www.sciencemag.org/features/2017/12/biomarkers-brain-puttingbiomarkers-together-betterunderstanding-nervous-system
11.Lewczuk, P., Ermann, N., Andreasson, U., Schultheis, C., Podhorna, J., Spitzer, P., Zetterberg, H. (2018). Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s research & therapy, 10(1), 71. doi:10.1186/s13195-018-0404-9.
12.Cheng, Z., Yin, J., Yuan, H., Jin, C., Zhang, F., Wang, Z., Xiao, S. (2018). Blood-Derived Plasma Protein Biomarkers for Alzheimer’s Disease in
Han Chinese. Frontiers in aging neuroscience, 10, 414. doi:10.3389/fnagi.2018.00414
13.Zvěřová M. (2018). Alzheimer’s disease and blood-based biomarkers – potential contexts of use. Neuropsychiatric disease and treatment, 14, 1877–1882. doi:10.2147/NDT.S172285.
14.Winston, C. N., Goetzl, E. J., Akers, J. C., Carter, B. S., Rockenstein, E. M., Galasko, D., Rissman, R. A. (2016). Prediction of conversion from mild
cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s & dementia (Amsterdam, Netherlands), 3, 63–72. doi: 10.1016/j.dadm.2016.04.001.
15.Santiago, J. A., Bottero, V., & Potashkin, J. A. (2018). Evaluation of RNA Blood Biomarkers in the Parkinson’s Disease Biomarkers Program. Frontiers in aging neuroscience, 10, 157. doi:10.3389/fnagi.2018.00157.
16.An, M., & Gao, Y. (2015). Urinary Biomarkers of Brain Diseases. Genomics, proteomics & bioinformatics, 13(6), 345–354. doi:10.1016/j.gpb.2015.08.005
17.Fukuhara K., Ohno A., Ota Y., Senoo Y., Maekawa K., Okuda H. (2013).NMR-based metabolomics of urine in a mouse model of Alzheimer’s disease: identification of oxidative stress biomarkers. J Clin Biochem Nutr., 52:133–138.
18.Peng J., Guo K., Xia J., Zhou J., Yang J., Westaway D. (2014). Development of isotope labeling liquid chromatography mass spectrometry for mouse urine metabolomics: quantitative metabolomic study of transgenic mice related to Alzheimer’s disease. J Proteome Res.,13:4457–4469.
19. Stephanie R. Shepheard, Joanne W, Cardoso M, Wikelendt L, Dinning J P, Chataway T, Schultz D, Benatar M, Rogers L M. (2017). Urinary p75ECD. Neurology, 88 (12) 1137-1143.
20.Farah, R., Haraty, H., Salame, Z., Fares, Y., Ojcius, D. M., & Said Sadier, N. (2018). Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomedical journal, 41(2), 63–87. doi:10.1016/j.bj.2018.03.004.
21. https://www.neurologytimes.com/tbi/saliva-new-tool-concussion-diagnosis
22. https://news.psu.edu/story/495094/2017/11/20/research/molecules-spitmay-be-able-diagnose-and-predictlength-concussions
23.Walton E. L. (2018). Saliva biomarkers in neurological disorders: A “spitting image” of brain health?. Biomedical journal, 41(2), 59–62. doi:10.1016/j.bj.2018.04.005.
24. Sabbagh, M. N., Shi, J., Lee, M., Arnold, L., Al-Hasan, Y., Heim, J., & McGeer, P. (2018). Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: preliminary findings. BMC neurology, 18(1), 155. doi:10.1186/s12883-018-1160-y.
25.Colligris, P., Perez de Lara, M. J., Colligris, B., & Pintor, J. (2018). Ocular Manifestations of Alzheimer’s and Other Neurodegenerative Diseases:
The Prospect of the Eye as a Tool for the Early Diagnosis of Alzheimer’s Disease. Journal of ophthalmology, 2018, 8538573. doi:10.1155/2018/8538573.
26.Lim K.H. J, Li Q, He Z, Vingrys A, Wong H.Y. V, Currier N, Mullen J, Bui B, Nguyen T.O. C, (2016). The Eye As a Biomarker for Alzheimer’s Disease. Front.Neurosci., 10, 536.