Aging
June 8, 2020Abstract1 vol 2 issue5
June 8, 2020
Abstract
The novel coronavirus disease (COVID-19) pandemic, caused by SARS-CoV-2, has affected people’s health in more than one way with catastrophic impacts on primary healthcare systems across the globe. However, the rapid expansion of knowledge over the last few months, supported by rigorous scientific research, has enabled our understanding of the mechanism(s) of COVID-19 and potential therapeutics. Initial infection of the upper respiratory tract by SARS-CoV-2 is mediated by binding of the virus to host expressed angiotensin-converting enzyme 2 receptor (ACE2). While viral pneumonia is one of the predominant manifestations of COVID-19, current evidence shows that the virus also affects multiple organs, especially the cardiovascular system. Comorbidities such as hypertension, diabetes, and coronary heart disease, not only result in worsening outcomes but also account for high death counts among infected patients. Serious cardiovascular complications such as cardiac injury, heart failure, and arrhythmias are often seen in the severe hospitalized cases of COVID-19; such cases predominantly feature hyper inflammation, cytokine storm along with elevated cardiac injury biomarkers. Whether viral invasion through ACE2—expressed in heart cells—is responsible for the development of new cardiovascular dysfunction in COVID-19 patients without any history of heart disease, remains under investigation. Other concerns have also surfaced regarding the use of renin-angiotensin system (RAS) inhibitors like angiotensin receptor blockers (ARBs) and ACE inhibitors (ACEIs), potentially increasing the severity of COVID-19. Here, we review current research and epidemiological data on SAR-CoV-2 infection and its clinical manifestations with a focus on cardiovascular complications and mechanisms of injury. Current guidelines over the use of RAS inhibitors in the context of the speculated role of ACE2 in COVID-19 patients are also discussed.
Introduction
Since the identification of novel coronavirus infection in a small cluster of pneumonia patients in Wuhan, China, SARS-CoV-2 (Severe Acute Respiratory Syndrome COronaVirus 2)—the virus responsible for COronaVIrus Disease (COVID-19)—has been on a global rampage killing more than 3 million people in its wake, stretching healthcare systems to their limits and causing severe socioeconomic loses. Even with stringent containment measures such as social distancing, rigorous disinfection, and lockdown, the transmission rates have been difficult to reduce. The US, which has been hit hard by the pandemic, saw an unprecedented rise in the number of COVID-19 cases between March and April, putting the nation on top for the highest number of cases1.
Since the outbreak, we have gathered a substantial amount of information about SARS-CoV-2 genetics and pathology. Genome sequencing and phylogenic analysis revealed that SARS-CoV-2 is a positive-stranded RNA β-coronavirus. It has 80% nucleotide homology to its ancestor SARS-CoV that caused the 2002 SARS outbreak and 50% nucleotide homology to MERS-CoV (Middle East Respiratory Syndrome Coronavirus) that was responsible for the 2012 outbreak2,3. Consequently, SARS-CoV-2 employs the same receptor to enter cells as SARS-CoV: the transmembrane Angiotensin-Converting Enzyme II (ACE2) receptor3. Once inside the upper respiratory tract, SARS-CoV-2 binds to ACE2 in the nasal epithelium through its surface spike protein (S), and through a serine protease cleavage event, is endocytosed into the host cell4. Tissue ACE2 expression is broad and occurs in a variety of cell types. In the respiratory system, it is predominantly expressed in the alveolar type II (AT2) cells of the lungs and to a variable extent in other cells like heart, kidneys, intestinal epithelium, and vascular endothelium4.
Clinical symptoms of SARS-CoV-2 are diverse and overlap with those associated with other viral respiratory infections; however, the rapid transmission rates combined with a higher risk of mortality distinguish COVID-19 cases from influenza. Symptomatic COVID-19 can range from mild symptoms like cough, fever, sore throat to severe manifestations like pneumonia, dyspnea, and acute pulmonary stress (ARDS) that often require admittance to hospital5. Most ICU cases of critically ill patients present with life-threatening conditions, such as acute cardiac injury, severe lymphopenia, shock, and abnormal inflammatory response that most often progress to multi-organ failure and death5,6,7. While a large proportion of COVID-19 infections are not severe, hospitalized cases commonly have a critical or fatal disease. In a report from the Chinese Center for Disease Control and Prevention that included 44,672 confirmed COVID-19 cases, the overall case fatality rate (CFR)—the number of deaths divided by the number of confirmed cases—was 2.3%, but in the critically ill population, the CFR rose to a staggering 49%8. In the worst affected countries, the CFR is anywhere between 6-16%9, and there is a strong correlation of mortality rate with advanced age6. In Italy, the CFR among patients aged 80 years or older rose to almost 20% owing to a greater proportion of geriatric population in the country (23% of the population >65)10. With more diagnostic tests starting to become available and accessible in recent times, the case fatality rate, which depends on the number of infected cases, is more likely to reflect an accurate estimation of the level of death in the COVID-19 pandemic.
Epidemiological and meta-analyses data from the first two months of the outbreak in China shows a consistent association of pre-existing cardiovascular disease (CVD) and relevant risk factors with the severity of COVID-19. A study on a pool of 1527 COVID-19 patients from six different hospitals in China reported both hypertension and diabetes to be twice as prevalent in ICU admitted COVID-19 cases than in their non-ICU counterparts11. In yet another early meta-analysis of seven studies, including 1576 COVID-19 cases, the most frequent comorbidities were hypertension (21.1%) and diabetes (9.7%), followed by cardiovascular disease (8.4%) and respiratory disease (1.5%)16. It is prudent to conclude, given the evidence, that pre-existing morbid ailments in COVID-19 patients contribute to an elevated risk of death2,14.
Do cardiovascular comorbidities increase mortality among COVID-19 patients?
The most common underlying health issues found to result in poorer prognosis and mortality among COVID-19 patients are hypertension, diabetes mellitus (DM), and coronary heart disease4,12,14. Comorbid patients often require intensive care treatments compared with those who have no underlying medical issues12. A large case-series report from the Chinese center for disease control and prevention, including 44,672 confirmed COVID-19 cases showed an increase in the mean death rate (2.3%) with comorbid conditions: 10.5% for cardiovascular disease, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension. Non-critical COVID-19 patients with no underlying comorbidities had no death13. As per reports from the National Health Commission of China, on COVID-19 patients who died, 35% had a history of hypertension, and 17% had a history of coronary heart disease15. A multi-center meta-analysis including a cohort of 1099 COVID-19 patients from 552 hospitals in China revealed that among the severely sick, 38.7% had comorbid health conditions, specifically, 23.7% with hypertension and 16.2% with diabetes. A high death rate of 8.1% in the severe cases vs. only 0.1% in the non-severe cases revealed that underlying cardiovascular conditions account for a large proportion of fatalities from COVID-1917.
Several mechanisms are thought to be responsible for the heightened susceptibility seen in severe COVID-19 infections with underlying CVD (Figure 1). Some of the possibilities could be: immune dysregulation due to an imbalance in type 1 and type 2 T helper cell response, insufficient tissue oxygenation during pulmonary distress, and adverse effects originating from treatment regimens that are yet to be evaluated in the setting of this pandemic18. As evidence suggests, SARS-Cov-2 seems to aggravate complications in patients with pre-existing cardiovascular disease, but does it induce new cardiac pathologies also?
Could SARS-CoV-2 infection be responsible for promoting new heart injuries?
Some initial case studies originating from China reported high levels of high-sensitivity cardiac troponin I (hs-cTnI) and troponin T (TnT) in critically ill COVID-19 patients, suggesting complications involving acute myocardial injury12,19. Hs-cTnI and TnT are biomarkers of acute cardiac injury and correlates well with other diagnostic markers of myocardial shock, such as high-sensitivity C-reactive protein and N-terminal pro-brain natriuretic peptide (NT-proBNP)18. In the aforementioned study reported by the National health commission of China, 11.8% of hospitalized COVID-19 patients, without underlying CVD, died of newly developed cardiac injury or cardiac arrest, as evidenced by their high levels of serum hs-cTnI15. A single-center case report including 187 patients with confirmed COVID-19 revealed that elevated TnT mediated myocardial injury claimed more lives than underlying CVD: a death rate of 37.5% was noted for those without CVD but with high TnT vs. 13.33% for those with underlying CVD and normal TnT20.
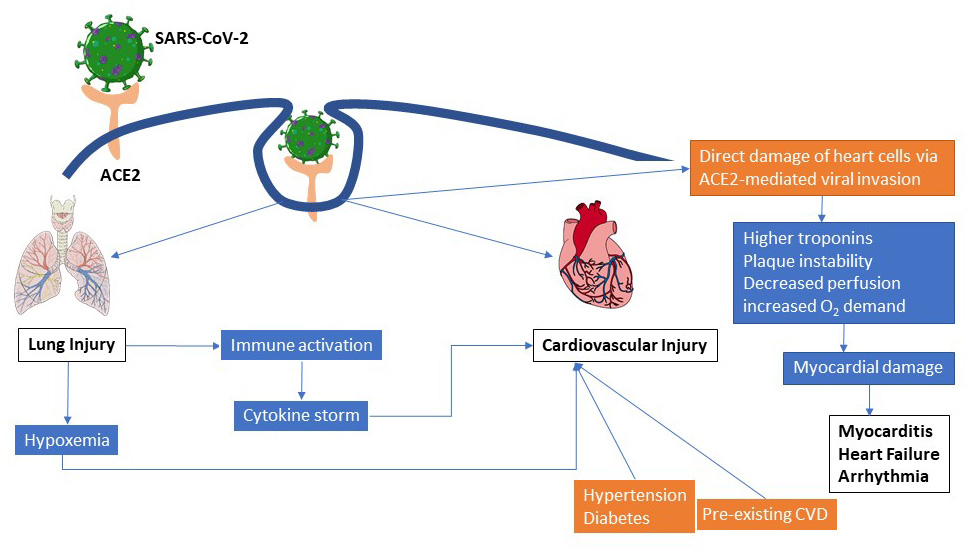
Figure 1: Potential pathways of COVID-19 associated pulmonary and cardiovascular injury. Infection of the respiratory tract by SARS-CoV-2 leads to systemic inflammation and dysregulated immune response which subsequently progresses into a “cytokine storm”. Similarly vial invasion of cardiac myocytes may directly lead to myocardial dysfunction, arrhythmias and eventually heart failure. Pre-existing conditions like hypertension and diabetes can further complicate viral disease outcomes.
Currently, there is not enough data on the pathophysiological mechanisms employed by SARS-CoV-2 in causing heart injury. Some speculation, however, correlates increased ACE2 expression on cardiomyocytes—that could lead to direct myocardial infection by SARS-CoV-2—with the myocardial injury that is often seen in severe COVID-196,18,21(Figure 1).
ACE2 and SARS-CoV-2—facts and speculations.
Myocardial complications in COVID-19 infections, along with the fact that SARS-CoV-2 uses ACE2 to enter host cells—which is highly expressed in the heart—have fueled debates among researchers on the possible impact of ACE2 on COVID-19 cases with new or existing CVD.
ACE2, a transmembrane peptidase is a component of the renin-angiotensin system (RAS); RAS is a complex endocrine cascade regulating blood pressure, fluid volume, and electrolyte balance. ACE2 is expressed in a variety of tissues, but at an organ level, the highest activity is seen in the upper GI tract (ileum) and kidney followed by heart, brain stem, lung, vascular endothelium, stomach, and mucosal surfaces4,22. ACE2 counters the deleterious activity of a hyperactive RAS that promotes hypertension, oxidative stress, and inflammation by converting ANG II (angiotensin II—a vasoconstricting octapeptide) to Ang-(1-7) [angiotensin-(1-7)—a vasodilating heptapeptide]. A higher level of ACE2 receptor showed improved cardiac function post myocardial infarction and better glycemic control in diabetes, possibly through the cardioprotective, anti-inflammatory, and anti-fibrotic actions of Ang-(1-7)22,23. ACE2 knock out mice developed severe lung injury24.
How ACE2 is modulated in the context of SARS-CoV-2 infection is not yet fully known. Studies on SARS-CoV revealed that viral infection reduced ACE2 in mice, leading to an increase in ANG II, which promoted pulmonary vascular permeability, inducing pulmonary edema, and lung dysfunction6. At present, preliminary research on SARS-CoV-2 yields similar data: virus-induced downregulation of ACE2. Either viral internalization by endocytosis with ACE2 reduces surface ACE222 or protease-mediated ACE2 shedding during the infection (identical to SARS-CoV infection) may bring down the level23. In either case, loss of ACE2 would disrupt the generation of cardioprotective Ang-(1-7), increasing the ratio of ANG II:Ang-(1-7) and, in theory, could potentiate pulmonary and cardiac damage in COVID-19 infections. Indeed, this could partially explain the cardiopulmonary manifestations, such as myocardial injury, respiratory dysfunction, and hyper-inflammation seen in COVID-19 patients23.
On the one hand, ACE2 is cardioprotective and prevents lung injury, and on the other, it serves as the portal of entry for SARS-CoV-2, then is ACE2 beneficial or detrimental in the current pandemic situation? Although definitive answers to that are lacking, researchers predict that a lower ACE2 in the nasal epithelium could help restrict SARS-CoV-2 infiltration in the body, but at the same time, a higher ACE2 in the lower respiratory tract and heart might help reduce SARS-CoV-2 -mediated risks of lung and myocardial injuries among vulnerable COVID-19 patients22,25.
ACE, a homolog of ACE2, converts ANG I (angiotensin I—a decapeptide pro-hormone) into ANG II and also degrades Ang-(1-7). By increasing ANGII, it exerts many deleterious effects on the cardiopulmonary system through stimulating myocyte hypertrophy, fibroblast proliferation, and aldosterone secretion that can potentiate cardiac fibrosis and inflammation. ACE inhibitors thus have been a standard of choice in treating heart failure patients and have shown promising effects on cardiac function and survival22.
Concerns have been raised that antihypertensive medications, such as ACE inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), could negatively impact the risk of SARS-CoV-2 infection and lead to severe COVID-19 owing to the role of ACE2 as the virus receptor (Figure 2). However, there are no clinical data currently available that support or reject this notion2,6,23. Research in animal models has demonstrated that ACEIs/ARBs upregulate ACE2 in heart and kidney26,27, raising speculations over increased susceptibility of ACEIs/ARBs consumers to SARS-CoV-2 infection or developing severe COVID-19. In contrast, blockade of angiotensin type I receptor (AT1R) in mice infected with SARS-CoV, protected against ANG II-induced acute lung injury and pulmonary edema28. Due to a lack of sufficient evidence in humans, American Heart Association (AHA) and other expert guidelines recommend against discontinuation of RAS inhibitors in CVD patients with COVID-19. Whether ACEIs/ARBs are worsening clinical outcomes by facilitating ACE2-mediated virus entry or protecting against virus-induced cardiac and pulmonary damage through increased Ang-(1-7) and decreased ANG II, remain to be seen (Figure 3). In fact, a couple of clinical trials with ARB Losartan (NCT04335123, NCT04312009) are underway in patients with COVID-19 to determine its efficacy in mitigating lung injury and hospitalization. Trials like this, along with observational studies, are needed to assess the complex interplay of SARS-CoV-2 with the RAS network and how that will aid in adapting treatment strategies for reducing the burden of COVID-19 among CVD patients.
Conclusions
As COVID-19 pandemic evolves in real-time, so is our understanding of the disease and its causative agent SARS-CoV-2. But, we still have long ways to go in terms of finding effective clinical measures and therapeutic paradigms for the treatment of COVID-19. Though severe respiratory illness remains a significant cause of mortality, pre-existing cardiovascular conditions particularly raise the risk of developing severe disease with poor prognosis. Virus-induced lymphocytopenia and hyper inflammation could promote the development of new-onset cardiac injury in COVID-19 patients, although the mechanisms remain poorly understood. Since there are no indications of any detrimental or beneficial effects of consuming ACEIs and ARBs on the susceptibility to SARS-CoV-2 and the severity of COVID-19, their usage should not be discontinued in patients with heart failure and other CVD. As new evidence emerges from large-scale clinical studies and retrospective analyses, it would be prudent to evaluate associations of current therapies in COVID-19 clinical outcomes and adopt tailored treatment strategies that will reduce the burden of disease in cardiovascular patients.
References:
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- Madjid, Mohammad, et al. “Potential effects of coronaviruses on the cardiovascular system: a review.” JAMA cardiology(2020).
- Zhou, Peng, et al. “A pneumonia outbreak associated with a new coronavirus of probable bat origin.” nature7798 (2020): 270-273.
- Cheng, Paul, et al. “Cardiovascular Risks in Patients with COVID-19: Potential Mechanisms and Areas of Uncertainty.” Current Cardiology Reports5 (2020).
- Wu, Chaomin, et al. “Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China.” JAMA internal medicine(2020).
- Clerkin, Kevin J., et al. “Coronavirus disease 2019 (COVID-19) and cardiovascular disease.” Circulation(2020).
- Zhou, Fei, et al. “Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.” The lancet(2020).
- Wu, Zunyou, and Jennifer M. McGoogan. “Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention.” Jama13 (2020): 1239-1242.
- Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/mortality
- Onder, Graziano, Giovanni Rezza, and Silvio Brusaferro. “Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy.” Jama(2020).
- Li, Bo, et al. “Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China.” Clinical Research in Cardiology(2020): 1-8.
- Huang, Chaolin, et al. “Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.” The lancet10223 (2020): 497-506.
- Wu, Zunyou, and Jennifer M. McGoogan. “Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention.” Jama13 (2020): 1239-1242.
- Sławiński, G., and Ewa Lewicka. “What should a cardiologist know about coronavirus disease 2019?.” Kardiologia Polska4 (2020): 278-283.
- Zheng, Ying-Ying, et al. “COVID-19 and the cardiovascular system.” Nature Reviews Cardiology5 (2020): 259-260.
- Yang, Jing, et al. “Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis.” International Journal of Infectious Diseases94 (2020): 91-95.
- Guan, Wei-jie, et al. “Clinical characteristics of coronavirus disease 2019 in China.” New England journal of medicine18 (2020): 1708-1720.
- Babapoor-Farrokhran, Savalan, et al. “Myocardial injury and COVID-19: Possible mechanisms.” Life Sciences(2020): 117723.
- Wang, Dawei, et al. “Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China.” Jama11 (2020): 1061-1069.
- Guo, Tao, et al. “Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19).” JAMA cardiology(2020).
- Adão, Rui, and Tomasz J Guzik. “Inside the heart of COVID-19.” Cardiovascular research 116,6 (2020): e59-e61.
- South, Andrew M., Debra I. Diz, and Mark C. Chappell. “COVID-19, ACE2, and the cardiovascular consequences.” American Journal of Physiology-Heart and Circulatory Physiology5 (2020): H1084-H1090.
- Gonzalez-Jaramillo, Nathalia et al. “The double burden of disease of COVID-19 in cardiovascular patients: overlapping conditions could lead to overlapping treatments.” European journal of epidemiology 35,4 (2020): 335-337.
- Imai, Yumiko et al. “Angiotensin-converting enzyme 2 protects from severe acute lung failure.” Nature 436,7047 (2005).
- Patel, Ankit B., and Ashish Verma. “Nasal ACE2 Levels and COVID-19 in Children.” JAMA.
- Ishiyama, Yuichiro, et al. “Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors.” Hypertension5 (2004): 970-976.
- South, Andrew M., et al. “Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic.” Nature Reviews Nephrology(2020): 1-3.
- Kuba, Keiji, et al. “A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury.” Nature medicine8 (2005): 875-879.