Non-Invasive Diagnostic Biomarkers for Alzheimer’s Disease
Dr. Shyamasri Biswas*
Chief Editor
Biotechnology Kiosk, USA
Abstract
Keywords: Alzheimer’s disease, Biomarkers, Non-Invasive, Mild Cognitive Impairment, Neuron
To cite this article: Biswas S, Non-Invasive Diagnostic Biomarkers for Alzheimer’s Disease, Biotechnol. kiosk, Vol 3, Issue 1, PP: 12-18 (2021); DOI: https://doi.org/10.37756/bk.21.3.1.2
*E-mail: shyabiswas@gmail.com; shyabiswas@biotechkiosk.com
It is estimated that Alzheimer’s disease (AD) could affect 75 million people in 2030 with the current trend of rapidly increasing aging population across the world with much of the increase expected to happen in developing countries [1]. Studies have strongly suggested that in AD, pathologic changes in the brain start well before any obvious symptoms of memory loss with amyloid beta (Aβ) pathology are noticed. This is thought to be a key initial step in the progression of AD, which is followed by the development of neurofibrillary tau pathology (Figure 1) [2]. For the characterization of the disease, three different stages are suggested that include preclinical (or asymptomatic) AD, mild cognitive impairment (MCI) due to AD and dementia due to AD. However, it is still a challenge to do an accurate premorbid diagnosis of the disease, which is currently based upon clinical presentations and other imaging and biofluid biomarkers [1]. With respect to the current state-of-the-art of AD diagnostics, positron emission tomography (PET) scans are known to be effective to reveal Aβ or tau accumulation in the brain. Further, magnetic resonance imaging (MRI) has also been employed to measure function and reveal brain atrophy. Excellent diagnostic accuracy can be achieved with specific cerebrospinal (CSF) biofluid constituents, such as amyloid beta 42 (Aβ42) that correlates with extracellular senile plaques. Such studies can be extended to total tau (T-tau) that reflects the intensity of neuronal damage and phosphorylated tau (P-tau) and correlates with tangle pathology. However, in all these cases, the relatively invasive nature of CSF collection poses challenge. The widespread use in routine primary care practice is limited due to the complex procedure. Further, in CSF collection process, a lumbar is punctured that can be unpleasant to the patient involved [1-4].
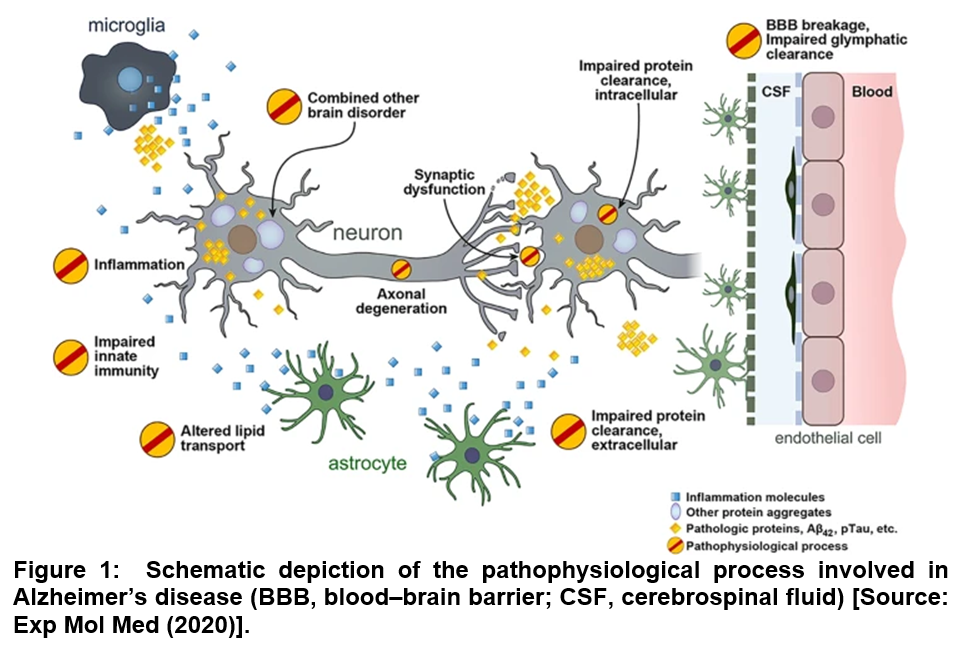
It can therefore be stated that diagnosing degenerative diseases of the nervous system such as AD can be a complicated process. Further, the entire method may not be comfortable to patients because of the involvement of expensive procedures such as image the brain, or invasive tests to assess cerebrospinal fluid by lumbar puncture. To overcome these challenges, a new line of thinking has emerged lately among AD researchers that seeks to develop non-invasive early diagnostic biomarkers that can not only be employed to monitor disease progression and manage care, but they will also provide an important and scalable mean of measuring clinical outcomes during AD therapies [1, 2].
One viable non-invasive diagnostic avenue could be blood tests that is believed to have the potential to detect disease before the patient presents with symptoms. This could be immensely helpful in cases where early interventions are deemed necessary to achieve the best treatment success. To this end, the published clinical studies showed measurement of variants of tau in the blood, which is a protein that normally functions to help stabilize microtubules within neurons. These studies demonstrated that a blood test measuring one of two phosphorylated forms of tau (p-tau181 or p-tau217) could effectively differentially diagnose AD from other neurodegenerative diseases with high accuracy and cost-effective manner compared to more costly and invasive standard diagnostics. These blood tests were also shown to be able to identify the disease at relatively early stage during cognitive decline [5-7]. However, these blood-based diagnostics are still far from being able to detect the disease at a presymptomatic stage, which is considered the key in AD therapy and its possible cure.
In addition to the more-widely used blood specimens, saliva based non-invasive diagnostic of AD has gained attention for some unique advantages. These include relative ease of collection that involves no experienced personnel, which essentially offers the exciting possibility for self-collected samples and completely non-invasive and inexpensive sample collection. Such sample collection does not require any anticoagulants along with it provides easier storage under the human tissue authority guidelines and permissions [1]. However, there are some challenges that include the decreased concentration of analytes. This requires more sensitive analytical approaches. In addition, inability of approximately 1/3 of participants to produce an adequate saliva sample also poses limitation. Salivary based studies have focused on the detection of the amyloid beta 42 (Aβ42) peptide by using an enzyme-like immunosorbent assay (ELISA) as their main experimental approach to detect Aβ. Researchers have suggested some other biomarkers besides Aβ and tau proteins that have shown potential as diagnostic means in saliva specimens. For example, lactoferrin, which is an antimicrobial peptide with a known Aβ-binding ability has been investigated that has shown significantly reduced lactoferrin in AD patients compared to healthy controls [1, 8, 9].
Researchers have suggested several hypotheses to explain the origin of salivary biomarkers that are indicative of AD. It has been proposed that biomarkers may be secreted by nerves into salivary glands because of their close proximity to the central nervous system. This also includes the salivary proteins that originate after transport of molecules from blood to saliva through ultrafiltration and passive diffusion or active transport [1]. Recent studies have shown the potential of oral microbiome as a diagnostic means for AD. A quantitative polymerase chain reaction (qPRC) assay was employed to quantify the load Porphyromonas gingivalis (P. gingivalis) for the development of chronic periodontitis that was considered as a high-risk factor for AD. It was found that a majority of CSF and all salivary samples were positive for the presence of P. gingivalis. These studies suggest potential use of P. gingivalis that may serve as a differential non-invasive diagnostic marker for AD [1, 10].
Another potential non-invasive specimen is oral mucosa cytology samples that have been suggested for an almost non-invasive and relatively inexpensive alternative for AD diagnosis. Researchers have studied buccal cells as biomarkers in different neurological disorders since these cells that are similarly to skin and brain cells can be derived from ectodermal tissue and therefore, they can be embryologically related to the central nervous system and share common AD-specific characteristics [11].
Future studies of AD-related pathophysiology through biomarkers are linked to diverse aspects of AD pathophysiology that include neurodegeneration, synaptic dysfunction, neuroinflammation, lipid dysmetabolism and disturbed protein clearance. It is believed that such biomarkers-based characterization would be immensely helpful for predicting the progression of individual facets of the pathology along with understanding their relative contributions to clinical deterioration [2]. For example, a common perspective among researchers is based on comprehensive understanding of disease status that is expected to aid in the selection of patients who are most likely to have a favorable response to specific disease-modifying therapies [2]. It is believed that such a characterization strategy could pave the way to superior treatment regimens that could allow monitoring of the response to treatment on an individual patient basis. This could be immensely beneficial given the diversity of AD pathology, and the developed precision medicine could increase the efficacy of the therapeutic effect (Figure 2) [2].
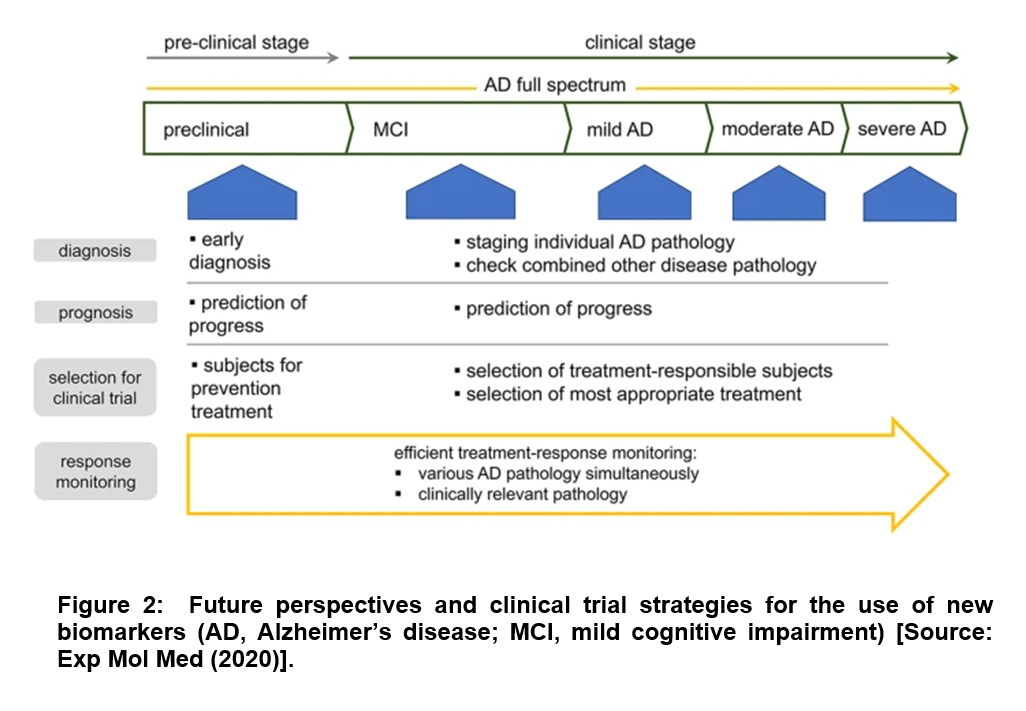
In conclusion, several studies have clearly suggested that loss of cognitive or motor capacity can be deeply concerning to those in early stages of neurodegenerative decline in Alzheimer’s disease (AD). This situation can get aggravated especially in the event of not being able to identify the cause of the onset of AD symptoms. This can be devastating to patients and their families because of the absence of answers and a clear diagnosis. With respect to blood serum biomarker research, it offers some hope to develop non-invasive accessible and affordable biomarkers with scalable tools to test and improve interventional therapies for AD. Regarding oral samples such as saliva or buccal cells for AD diagnosis, large-cohort studies are required to be conducted to ensure that these non-invasive oral samples can act as a research and/or clinical tool for AD.
References
[1] Paraskevaidi M, Allsop D, Karim S, Martin FL, Crean S. Diagnostic Biomarkers for Alzheimer’s Disease Using Non-Invasive Specimens. J Clin Med. 2020;9(6):1673, doi: https://doi.org/10.3390/jcm9061673.
[2] Park, S.A., Han, S.M. & Kim, C.E. New fluid biomarkers tracking non-amyloid-β and non-tau pathology in Alzheimer’s disease. Exp Mol Med. 2020;52, 556, doi: https://doi.org/10.1038/s12276-020-0418-9
[3] Aisen P.S., Cummings J., Jack C.R., Morris J.C., Sperling R., Frölich L., Jones R.W., Dowsett S.A., Matthews B.R., Raskin J. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017;9:60. doi: https://doi.org/10.1186/s13195-017-0283-5.
[4] Lashley T., Schott J.M., Weston P., Murray C.E., Wellington H., Keshavan A., Foti S.C., Foiani M., Toombs J., Rohrer J.D. Molecular biomarkers of Alzheimer’s disease: Progress and prospects. Dis. Model. Mech. 2018;11:dmm031781. doi: https://doi.org/10.1242/dmm.031781.
[5] Zetterberg H. Blood-based biomarkers for Alzheimer’s disease—An update. J. Neurosci. Methods. 2019;319:2–6. doi: https://doi.org/10.1016/j.jneumeth.2018.10.025.
[6] Thomas K Karikari, Tharick A Pascoal, Nicholas J Ashton, Shorena Janelidze, Andréa Lessa Benedet, Juan Lantero Rodriguez, Mira Chamoun, Melissa Savard, Min Su Kang, Joseph Therriault, Michael Schöll, Gassan Massarweh, Jean-Paul Soucy, Kina Höglund, Gunnar Brinkmalm, Niklas Mattsson, Sebastian Palmqvist, Serge Gauthier, Erik Stomrud, Henrik Zetterberg, Oskar Hansson, Pedro Rosa-Neto, Kaj Blennow, Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts, The Lancet Neurology, 2020;19(5):422, doi: https://doi.org/10.1016/S1474-4422(20)30071-5.
[7] Sebastian Palmqvist, Shorena Janelidze, Yakeel T. Quiroz, et al, Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders, JAMA. 2020;324(8):772-781. doi: https://doi.org/10.1001/jama.2020.12134.
[8] Sabbagh M.N., Shi J., Lee M., Arnold L., Al-Hasan Y., Heim J., McGeer P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol. 2018;18:1–4. doi: https://doi.org/10.1186/s12883-018-1160-y.
[9] Carro E., Bartolomé F., Bermejo-Pareja F., Villarejo-Galende A., Molina J.A., Ortiz P., Calero M., Rabano A., Cantero J.L., Orive G. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017;8:131–138. doi: https://doi.org/10.1016/j.dadm.2017.04.002.
[10] Liu X.-X., Jiao B., Liao X.-X., Guo L.-N., Yuan Z.-H., Wang X., Xiao X.-W., Zhang X.-Y., Tang B.-S., Shen L. Analysis of Salivary Microbiome in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2019;72:633–640. doi: https://doi.org/10.3233/JAD-190587.
[11] François M., Leifert W., Martins R., Thomas P., Fenech M. Biomarkers of Alzheimer’s disease risk in peripheral tissues; focus on buccal cells. Curr. Alzheimer Res. 2014;11:519–531. doi: https://doi.org/10.2174/1567205011666140618103827.