Perspective
Optimization of Bio-Electrochemical Systems
Abhijit Biswas
USA Prime Biotech LLC, 1330 NW 6th St, Suite A-2, Gainesville, FL 32601, USA
Abstract
The ongoing research in the sustainable energy sector has shown tremendous potential of microbes or electro-active biofilms (EABfs). These biofilms act as the important component of bioprocessing technologies that are based on bio-electrochemical systems (BESs). EABfs exhibit unique characteristics including redox reactions and resilience against otherwise harmful products that make BESs promising for important applications in energy recovery in the form of electricity or hydrogen or even production of fuels or chemicals from CO2. A deeper understanding of the mechanisms of EABfs characteristics is considered essential for the optimization of BESs for practical applications. To this end, a wide range of characterization techniques based on electrochemical, visual and chemical methods have been employed for the analyses of EABfs. These techniques can provide very valuable and wide-ranging information about EABfs that include performance, morphology and biofilm composition. Especially, significant attention has been paid to developing non-destructive visual techniques for EABfs characterization. The goal is to obtain in-situ information of EABfs functioning for industrial-scale development of BESs. Visual techniques are considered extremely useful for EABfs monitoring studies that can complement the information obtained with other characterization techniques. In this perspective, we have provided a short overview of various visual characterization techniques that have been proposed to study EABfs for the optimization of BESs.
Keywords
Bio-electrochemical systems; electro-active biofilms; characterization; visual techniques
Received: August 25, 2021
Revised Manuscript: September 15, 2021
Accepted: September 20, 2021
*E-mail: abbtf@yahoo.com
1. Introduction
The dual challenge of rapidly rising world population with increasing energy demands and simultaneous depletion of fossil fuel reserves has accelerated research and developments in sustainable energy and recovery technologies that include recovery of energy and nutrients from wastewater [1, 2]. To this end, bio-electrochemical systems (BESs) have been shown very promising to recover resources such as nutrients and energy from wastewater. BESs employ microorganisms and the microbes that act as catalysts can use external electron acceptors or electron donors as electrodes for chemical conversions [3-5]. Hence, BESs are capable of converting chemical energy into electrical energy (and vice-versa). BESs can be broadly divided into two major sustainable energy technology platforms – microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) (Figure 1) [6].
Previous studies showed the potential of MFCs that were employed to convert organic wastes including low-strength wastewaters and lignocellulosic biomass into electricity. On the other hand, MECs were employed where electrical energy was used to produce hydrogen or other industrially useful products including caustic and peroxide. In addition, researchers also designed and implemented BESs to recover nutrients, metals or removal of recalcitrant compounds. Furthermore, solar energy was used to generate electricity by implementing photosynthetic micro-organisms along with higher plants. Thus, it is possible to realize a range of potential applications of BESs by utilizing the diversity on microbial and enzymatic catalysts that are offered by nature (Figure 1) [6].
In addition to MFCs and MECs for energy recovery in the form of electricity or hydrogen, another type of energy technology platform has been considered, which is based on Microbial Electrosynthesis Cell (MES) (Figure 1) [6]. MES system is used for the production of fuels or chemicals from CO2. All these technologies are governed by the working principle on electro-active microbial communities (such as electro-active biofilms). However, the difference in these technologies is that MFCs and MECs operate with exoelectrogens at the anode; whereas, MESs uses eletrotrophs at the cathode for operation [7-9].
BESs are made of electro-active biofilms (EABfs). These biofilms are essentially electro-active bacteria that develop on the surface on an electrode. During the conversion of chemical energy into electrical energy and vice versa, the electro-active bacteria acts as catalysis to promote the energy conversion. Since these biofilms play extremely important roles in BESs formation and operation, the current research has focused on studying the required operating conditions for bio-catalysis of EABfs. For the improvement of BESs performance and optimization, there have been studies on employing advanced materials and optimized electrode designs for desired interaction between EABfs and electrode surface. It is not only important to gain insights into the behavior of electro-active bacteria and EABfs with respect to the operational conditions that include electrode designs and electrode current and potentia, but also characterization studies are considered essential to better understand the mechanisms of EABfs [10-15].
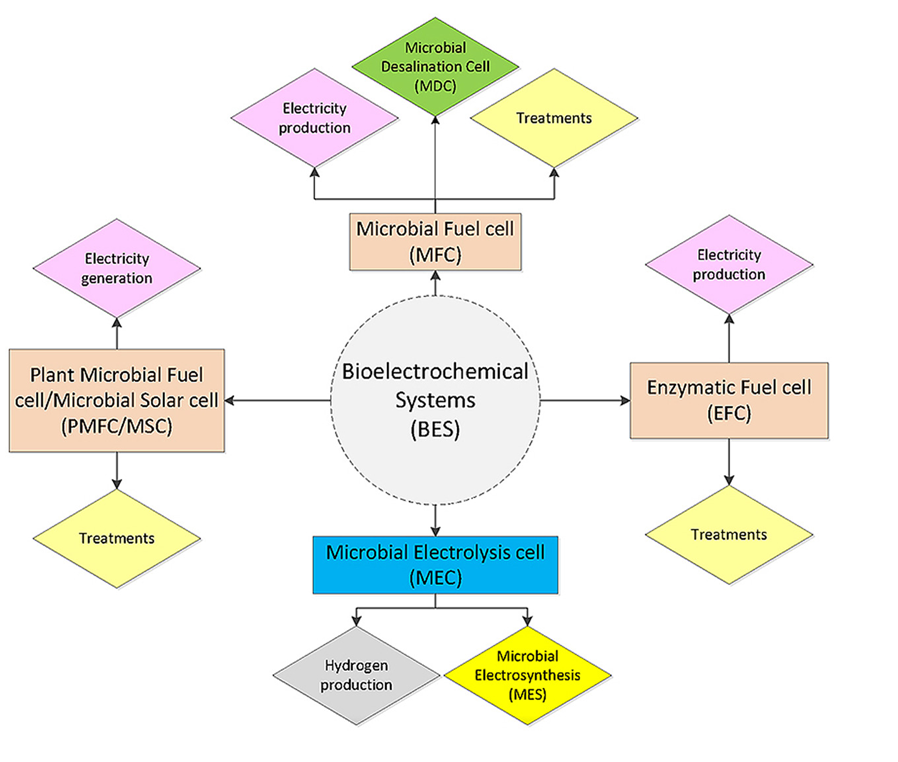
Figure 1: Bio-electrochemical systems and various applications [Source: Renewable Energy (2016)].
2. Important Connection between Electro-Activity and Biofilm Characteristics
Various studies have indicated that it is critically important to establish the relationship between electroactivity and biofilm characteristics for the optimization of BESs. This is due to the reason that increased electroactivity has been found to result in efficient product formation in all BES operations. Therefore, a significant attention has been paid to generate new avenues that enable modifying the electrode surface to enhance cellular attachment by an increased surface area. In addition, more robust biofilm formation by engineering strains is also suggested. All these efforts are aimed at enhancing biofilm formation on electrodes. It is believed that efficient design and optimization of BESs can be achieved by fine-tuning bio-electrochemical cell parameters. To this end, various research tools such a mathematical modeling that allows for scalability and investigation of microbe–electrode interactions and the effect of electrode type have been proposed. Additionally, electrode modifications, microbial composition along with understanding the cellular processes underlying electron exchange and product formation have been considered in all current and future research directions to optimize BESs for practical applications. Especially, research efforts are directed to improving Coulombic efficiency and optimizing biofilm formation along with bioprospecting for electroactive strains and electron exchange mechanisms. These efforts are expected to impact the development of optimization of BESs in the future [16-20].
3. Characterization of Electro-Active Biofilms: Conventional Techniques
Several types of conventional characterization techniques have been employed for the in-depth analysis of EABfs. These techniques can be employed for electrochemical, visual, and chemical analyses to study EABfs [21]. A wide range of information about EABfs including knowledge about microbial activity, biofilm structure, thickness and composition, mass transfer limitations and also conductivity can be obtained by employing these techniques [22-24]. For example, researchers have employed electrochemical techniques to determine the general performance indicators of EABfs to reveal the relationship between electric current and potential. Some commonly used electrochemical techniques include Cyclic Voltammetry (CV), potentiostatic control, and Electrochemical Impedance Spectroscopy (EIS). These electrochemical techniques have been employed to study different stages of EABfs growth. This also includes obtaining information about microbial activity, information about redox active compounds along with charge storage. Researchers have also shown employability of chemical analyses in BESs primarily to estimate the concentration of substrate along with products in the bioreactor. Establishing the connection between these concentrations and the electrons exchanged at the electrode(s) is very important as it gives insights into the coulombic efficiency of electrodes [25-28]. Despite some usefulness of these conventional characterization methods, these techniques are usually destructive that often lead to the destruction of biofilm after performing a given analysis. It makes extremely difficult to monitor EABfs during the experiments [29]. We will discuss in the following section some relevant non-destructive visualization techniques for EABfs.
4. Non-Destructive Modern Visualization Techniques for Biofilm Characterization and Monitoring
It is known that EABfs are biological matrixes that comprise embedded electro-active bacteria. In this scenario, the biofilm composition and mechanical properties are usually unique that pose serious challenge to characterize and study these films. This is due to the reason that as EABfs grow on an electrode, the matrix composition constantly changes with time. This makes characterizing EABfs and predicting their performance very difficult. To overcome these challenges, researchers have proposed visual techniques that can be used for multiple functions. These include detecting specific compounds that are present in the extracellular matrix and also to visualize the distribution of the biofilms as a function of time. Visual techniques can be leveraged to image the morphology and cellular density in the biofilm structure. The major advantage is the ability to gain insights into the 3D distribution of biofilms. This enables identifying species that comprise the biofilm and also mapping their disposition in the biofilm. Particularly, the combination of electrochemical and visual techniques is considered ideal that allows to obtain new information about EABfs. Furthermore, it is believed that in-situ visualization techniques for EABfs can be leveraged to perform monitoring of biofilm characteristics, and follow the growth of biofilm over time. To this end, some recognized visual techniques including Confocal Laser Scanning Microscopy (CLSM) and Optical Coherence Tomography (OCT) have been shown very useful to study EABfs along with monitoring film thickness, composition to localize microbial species [30, 31].
In addition to CLSM and OCT, there are some other visual techniques that have been shown in some studies. These include Raman Microscopy, Scanning Electron Microscopy (SEM), Scanning Transmission X-ray microscopy (SXRM) and Magnetic Resonance Imaging (MRI) that have been employed to obtain valuable information on electrochemical data. Among various visualization methods, CLSM is considered very promising and useful for visualization, quantification, 3D imaging along with characterization of biofilm composition. MRI and OCT are also frequently employed that allow for 3D imaging. These techniques have the ability to determine the biofilm distribution and its volume without destroying the sample. In addition, Raman and STXM are also non-destructive methods for studying the biofilm composition [32-34].
Recently, light sheet fluorescence microscopy (LSFM) was shown for nondestructive, label-free and in-vivo imaging of large electro-active biofilm specimen. The technique was shown to function even at nontransparent surfaces. In this study, researchers demonstrated LSFM for label-free analyses of prokaryotes on electroactive biofilms (Figure 2) [31]. Biofilm growth was linked to the production of current serving as measure of metabolic activity in-vivo. This was done by monitoring with high spatial and temporal resolution. After 35 h of exponential growth, they showed a growth of homogeneous biofilm with a thickness of 9 μm. Subsequently, a stratification of the biofilm including the formation of 3D structures was conducted for several hours. During this process, light reflection was shown to be sufficient that was used to visualize the biofilm structure and development over time. Fluorescence staining was employed to confirm the final morphology [31].
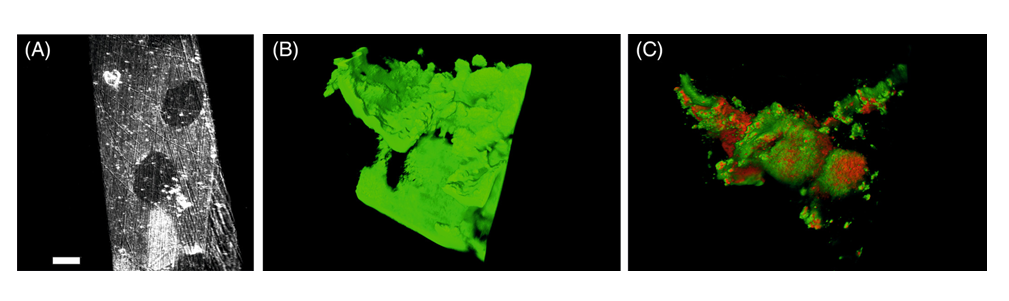
Figure 2: (A) Visualization of growth substratum is shown without any biofilm. (B) A comparison to a three-dimensional adult biofilm based on light reflection is shown. (C) Reanalysis of the same biofilm sample is shown after LIVE/DEAD nucleic acid fluorescent staining confirming the organization of the individual cells. The three-dimensional organization of the biofilm can be seen in reflection [Source: Cytometry Part A (2020)].
5. Conclusion
Bio-electrochemical systems (BESs) offer tremendous potential in today’s sustainable energy sector. Optimization of BESs is hugely important research field that involves characterization to understand the formation of electro-active biofilms that are integral components of BESs. To this end, several very promising characterization techniques for electro-active biofilm visualization and 3D imaging on an electrode have been proposed. Such characterization of biofilms is considered critical to optimize BESs technology and take the field of sustainable energy to the next level. Additionally, combinatorial approaches including conventional characterization of biofilms and non-destructive visualization methods could pave the way to new breakthroughs in electro-active biofilms in the future, which could significant benefit the BESs technology in the future.
References
[1] Borole A.P., Reguera G., Ringeisen B., Wang Z.W., Feng Y., Kim B.H.,
Electroactive biofilms: current status and future research needs,
Energy Environ. Sci., 2011; 4:4813-4834, DOI: https//doi.org/ 10.1039/c1ee02511b.
[2] Aziz, N.I.H.A., Hanafiah, M.M., Gheewala, S.H., Ismail, H. Bioenergy for a cleaner future: A case study of sustainable biogas supply chain in the Malaysian energy sector, Sustainability, 2020; 12(3213):1-24, DOI: https://doi.org/10.3390/su12083213.
[3] Das D., Microbial fuel cell: a bioelectrochemical system that converts waste to watts, 2017, ISBN: 978-3-319-66793-5, DOI: https://doi.org/10.1007/978-3-319-66793-5.
[4] Kiran R., Patil S.A., Microbial electroactive biofilms,
ACS Symp. Ser., 2019, 1323:159-186, DOI: https://doi.org/10.1021/bk-2019-1323.ch008.
[5] Santoro C., Arbizzani C., Erable B., Ieropoulos I., Microbial fuel cells: from fundamentals to applications, A review, J. Power Sources, 2017, 356:225-244, DOI: https://doi.org/10.1016/j.jpowsour.2017.03.109.
[6] Bajracharya Suman, Sharma Mohita, Mohanakrishna Gunda, Dominguez Xochitl, David Benneton, Strik P.B.T.B., Sarma Priyangshu M., Pant Deepak,
An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond, Renewable Energy, 2016, 98:153-170,
DOI: https://doi.org/10.1016/j.renene.2016.03.002.
[7] Logan B.E., Hamelers B., Rozendal R., Schröder U., Keller J., Freguia S.,
Aelterman P., Verstraete W., Rabaey K., Microbial fuel cells: methodology and technology, Environ. Sci. Technol., 2006, 40 (17):5181–5192, DOI: https://doi.org/10.1021/es0605016.
[8] Logan B.E., Rossi R., Ragab A., Saikaly P.E., Electroactive microorganisms in bioelectrochemical systems, Nat. Rev. Microbiol., 2019, 17:307-319, DOI: https://doi.org/10.1038/s41579-019-0173-x.
[9] Zheng T, Li J, Ji Y, Zhang W, Fang Y, Xin F, Dong W, Wei P, Ma J, Jiang M. Progress and Prospects of Bioelectrochemical Systems: Electron Transfer and Its Applications in the Microbial Metabolism. Front Bioeng Biotechnol., 2020, 8:10, DOI: https://doi.org/10.3389/fbioe.2020.00010.
[10] Erable B., Duţeanua N.M., Ghangrekar M.M., Dumas C., Scott K.,
Application of electro-active biofilms, Biofouling, 2010, 26:57-71, DOI: https://doi.org/10.1080/08927010903161281.
[11] Choi S., Chae J., Optimal biofilm formation and power generation in a micro-sized microbial fuel cell (MFC), Sensors Actuators A Phys., 2013, 195:206-212, DOI: https://doi.org/10.1016/j.sna.2012.07.015.
[12] Hindatu Y., Annuar M.S.M., Gumel A.M., Mini-review: anode modification for improved performance of microbial fuel cell, Renew. Sust. Energ. Rev., 2017, 73:236-248, DOI: https://doi.org/10.1016/j.rser.2017.01.138.
[13] Lee H.S., Electrokinetic analyses in biofilm anodes: Ohmic conduction of extracellular electron transfer, Bioresour. Technol., 2018, 256:509-514, DOI: https://doi.org/10.1016/j.biortech.2018.02.002.
[14] Caizán-Juanarena L., Krug J.R., Vergeldt F.J., Kleijn J.M., Velders A.H., Van As H., Ter Heijne A., 3D biofilm visualization and quantification on granular bioanodes with magnetic resonance imaging, Water Res., 2019, 167, DOI: https://doi.org/10.1016/j.watres.2019.115059.
[15] Chong P., Erable B., Bergel A., Effect of pore size on the current produced by 3-dimensional porous microbial anodes: a critical review
Bioresour. Technol., 2019, 289:121641, DOI: https://doi.org/10.1016/j.biortech.2019.121641.
[16] Babauta J, Renslow R, Lewandowski Z, Beyenal H. Electrochemically active biofilms: facts and fiction. A review. Biofouling., 2012, 28(8):789-812, DOI: https://doi.org/10.1080/08927014.2012.710324.
[17] Czerwińska-Główka Dominika, Krukiewicz Katarzyna, A journey in the complex interactions between electrochemistry and bacteriology: From electroactivity to electromodulation of bacterial biofilms, Bioelectrochemistry, 2020, 131:107401, DOI: https://doi.org/10.1016/j.bioelechem.2019.107401.
[18] Yadav, S., Patil, S.A. Microbial electroactive biofilms dominated by Geoalkalibacter spp. from a highly saline–alkaline environment. npj Biofilms Microbiomes, 2020, 6:38, DOI: https://doi.org/10.1038/s41522-020-00147-7.
[19] ter Heijne A., Pereira M.A., Pereira J., Sleutels T., Electron Storage in Electroactive Biofilms, Trends in Biotechnology, 2021, 39(1):34-42, DOI: https://doi.org/10.1016/j.tibtech.2020.06.006.
[20] Atnafu, T., Leta, S. New fragmented electro-active biofilm (FAB) reactor to increase anode surface area and performance of microbial fuel cell., Environ Syst Res, 2021, 10: 31, DOI: https://doi.org/10.1186/s40068-021-00234-4.
[21] Doyle L.E., Marsili E., The Dynamics and Characterization of Electroactive Biofilms,
Editor(s): Klaus Wandelt, Encyclopedia of Interfacial Chemistry,
Elsevier, 2018, 524-528, ISBN 9780128098943, DOI:
https://doi.org/10.1016/B978-0-12-409547-2.14040-5.
[22] Bartosch S., Mansch R., Knotzch K., Bock E., CTC staining and counting of actively respiring bacteria in natural stone using confocal laser scanning microscopy, J. Microbiol. Methods, 2003, 52:75-84, DOI: https://doi.org/10.2307/1882432.
[23] Lusk B.G., Parameswaran P., Popat S.C., Rittmann B.E., Torres C.I.,
The effect of pH and buffer concentration on anode biofilms of Thermincola ferriacetica, Bioelectrochemistry, 2016, 112:47-52, DOI: https://doi.org/10.1016/j.bioelechem.2016.07.007.
[24] Pepè Sciarria T., Arioli S., Gargari G., Mora D., Adani F., Monitoring microbial communities’ dynamics during the start-up of microbial fuel cells by high-throughput screening techniques, Biotechnol. Rep., 2019, 21:e00310, DOI: https://doi.org/10.1016/j.btre.2019.e00310.
[25] He Z., Mansfeld F., Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies, Energy Environ. Sci., 2009, 2:141-240, DOI: https://doi.org/10.1039/b814914c.
[26] Strycharz S.M., Malanoski A.P., Snider R.M., Yi H., Lovley D.R., Tender L.M.,
Application of cyclic voltammetry to investigate enhanced catalytic current generation by biofilm-modified anodes of Geobacter sulfurreducens strain DL1 vs. variant strain KN400, Energy Environ. Sci., 2011, 4:896-913, DOI: https://doi.org/10.1039/c0ee00260g.
[27] de Smit S.M., Buisman C.J.N., Bitter J.H, Strik D.P.B.T.B., Cyclic voltammetry is invasive on microbial electrosynthesis, ChemElectroChem, 2021, 8:3384-3396, DOI: https://doi.org/10.1002/celc.202100914.
[28] ter Heijne A., Schaetzle O., Gimenez S., Navarro L., Hamelers B., Fabregat-Santiago F., Analysis of bio-anode performance through electrochemical impedance spectroscopy, Bioelectrochemistry, 2015, 106:64-72, DOI: https://doi.org/10.1016/j.bioelechem.2015.04.002.
[29] Pereira J., Mediayati Y., van Veelen H.P.J., Temmink H., T. Sleutels, B. Hamelers, A. ter Heijne, The effect of intermittent anode potential regimes on the morphology and extracellular matrix composition of electro-active bacteria, Biofilm, 2021, 4:100064, DOI: https://doi.org/10.1016/j.bioflm.2021.100064.
[30] Azeredo J., Azevedo N.F., Briandet R., Cerca N., Coenye T.,
Costa A.R. , Desvaux M., DiBonaventura G., Hébraud M., Jaglic Z., Kačániová M., Knøchel S., Lourenço A., Mergulhão F., Meyer R.L., Nychas G., Simões M., Tresse O., Sternberg C., Critical review on biofilm methods, Crit. Rev. Microbiol., 2017, 43:313-351, DOI: https://doi.org/10.1080/1040841X.2016.1208146.
[31] Koch C, Kuchenbuch A, Marosvölgyi M, Weisshart K, Harnisch F. Label-Free Four-Dimensional Visualization of Anaerobically Growing Electroactive Biofilms. Cytometry A., 2020, 97(7):737-741, DOI: https//doi.org/10.1002/cyto.a.24169.
[32] Hu Z., Hidalgo G., Houston P.L., Hay A.G., Shuler M.L., Abruña H.D.,
Ghiorse W.C., Lion L.W., Determination of spatial distributions of zinc and active biomass in microbial biofilms by two-photon laser scanning microscopy,
Appl. Environ. Microbiol., 2005, 71:4014-4021, DOI: https://doi.org/10.1128/AEM.71.7.4014-4021.2005.
[33] Li C., Felz S., Wagner M., Lackner S., Horn H., Investigating biofilm structure developing on carriers from lab-scale moving bed biofilm reactors based on light microscopy and optical coherence tomography, Bioresour. Technol., 2016, 200:128-136, DOI: https://doi.org/10.1016/j.biortech.2015.10.013.
[34] Zhang P., Chen Y., Qiu J., Dai Y., Feng B., Imaging the microprocesses in biofilm matrices, Trends Biotechnol., 2019, 37:214-226, DOI: https://doi.org/10.1016/j.tibtech.2018.07.006.