
Introduction
Failure is part of scientific experiment and research, however, sometimes the price one has to pay for a failure might dilute the rewards of success altogether. Researchers in the field of predictive toxicology and drug development face failures as opposed to success most of the time. The past two decades has witnessed a steady rise in the rate of clinical trial failures that has led to increased healthcare costs. It takes nearly 10-12 years to develop a single potent drug with a monetary investment worth 2.5 billion dollars [1]. These numbers are taxing to the drug industry and cannot be ignored. Therefore, the diagnostic window needs to be shortened so that resources can be spent judiciously on effective treatment. This has led to the idea of “fail early and fail cheaply” within the pharmaceutical industry. Rapid, fast paced, reliable, cost effective and reproducible predictive models of human physiology are the need of the hour.
Predictive toxicology and bio-safety assessment of each and every chemical entity present in the environment is crucial as every chemical substance can be toxic or effective at a particular concentration/dose. The same applies to drugs, which are intended for therapeutic purposes but can be toxic and life threatening if not profiled stringently for their potential side effects and secondary targets. When the human body is subjected to a toxic insult, the response elicited is not restricted to one organ or one gene. A complex interplay of organ-organ interaction and gene-gene interaction takes place. Profiling these interactions in the conventionally advanced three-dimensional (3D) culture system and organoid culture is not feasible. Stem cell derived organoids have been instrumental in studying the molecular mechanisms and pathophysiology of diseases and find widespread application in regenerative medicine. However, an isolated organoid is not capable of mimicking the complex physiology that exists within a human
body and the absence of vasculature further restricts the profiling of drug metabolism. “Organ-on-a-chip” technology is the emerging trend in the field of clinical/medical research that has partially brought the long-standing financial feud to an end and has unlocked completely new avenues.
“Organ-on-a-chip” is simply the amalgamation of software designing, microfluidics, 3D organotypic systemculture and tissue engineering (Figure 1).
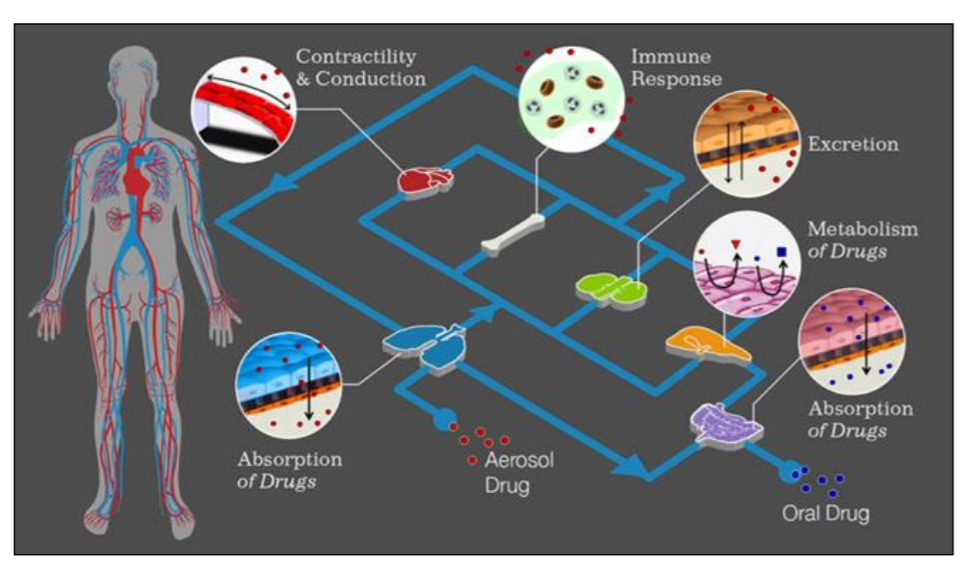
Figure 1: Concept of ‘organ- on-chip’ devices [Source: Findings from Wyss Institute, DARPA and IBM joint ventures 2015]
What are “Organ-on-a-chip” devices?
Research and development sectors and multidisciplinary team of collaborators have successfully engineered microfluidic culture devices that can easily mimic the physiology of an organ as well as the cellular micro architecture. Computer microchip manufacturing methods coupled with mechanobiology have been employed to produce these micro devices representing miniature human organs such as kidney, intestine, lung, bone marrow, skin etc. [2]. The basic design of an organ chip comprises a computer memory stick sized clear flexible polymer that contains hollow microfluidic channels. These channels are lined by human organ-specific living cells that are interfaced with human endothelial cell-lined artificial vasculature. In order to mimic the physical microenvironment of living organs such as peristaltic movement in the intestine and breathing motions in lung, artificial mechanical forces are applied. In addition to create artificial blood flow and mimic the dynamic nutrient distribution externally,
controlled microfluidics is applied.
These micro devices represent the three-dimensional cross-sections of major functional units of complete living organs. The most common material used for the microfluidic device fabrication is Polydimethylsiloxane (PDMS) but its applicability is restricted due to its property of absorption of hydrophobic drugs. To overcome this limitation tissue culture grade plastics such as styrene-ethylene/butylenestyrene copolymers, polystyrene and Polymethyl methacrylate (PMMA) have been used as suitable alternatives. These chips are further integrated with noninvasive biosensors that can potentially monitor cell behavior, receive stimuli and can perceive elicited drug response. By interconnecting different organ chips into one micro device, it is possible to simulate and recapitulate the complex multi-organ system functionality that exists within a human body. This advanced technology shall enable the screening and profiling of drug metabolism and pharmacokinetics (Figure 2) [3].
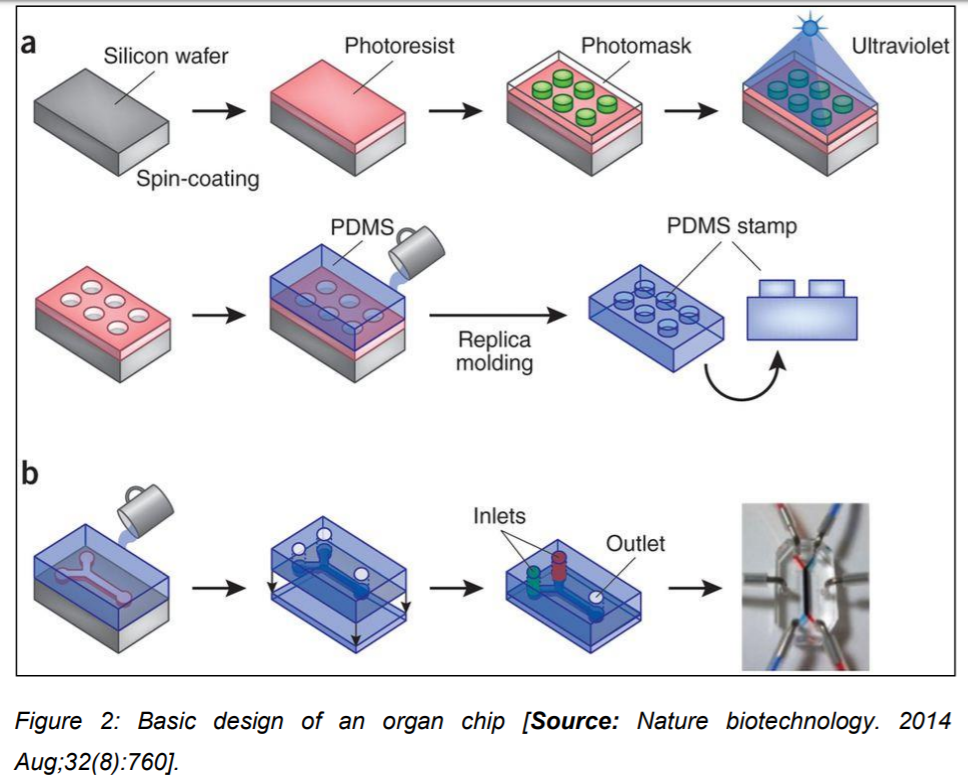
What are the– applications of “Organ on chip” devices?
Equipped with the ability to integrate the different tissue types that make up human organs, these micro devices offer an ideal system for studying
cellular and molecular mechanisms of disease, modeling species-specific disease states and even serve to identify novel therapeutic targets. Scientists have successfully created therapeutically pertinent interfaces such as the blood brain barrier and the alveolar-capillary interface with the help of these organ chips [1]. These devices are routinely employed to model diseases such as cystic fibrosis, neuropsychiatric disorders, study microvascular obstructions and compare various organ functions such as those of lung and kidneys. Another potent application is to study the effect of microbial influence on health and disease by culturing living microbiomes in direct contact with living human intestinal cells over an extended period to study intestinal infections or culturing influenza virus particles with lung epithelial cells to model lung infections. Organ chips have also been used to
investigate environmental factors such as cigarette smoke’s detrimental effect on tissue health and patient physiology. For this purpose human smoking behavior is mimicked by a smoking machine, which directly pumps cigarette smoke into the airspace of a human ‘Lung Airway Chip’ and the impact on human lung airway functions can be effectively modeled in-vitro. One of the pioneers in this field of organ on chip technologies has been the Wyss Institute of Harvard University where a team of researchers have successfully developed an integrated device which couples the functioning of various organs within a single chip and mimics the complexity of a multi organ system vasculature by circulating a fluid between their common vascular channels. This unique device has combined the physiological and biochemical functioning of ten different organs into one and is an efficient mimic of the whole-body physiology. Equipped with controlled artificial fluid flow and cell viability the device allows for a real-time monitoring of the cultured cells and organ tissues. This holistic approach has led to the formulation of “human Body-on-Chips” devices that can be used as predictive models of pharmacokinetic and pharmacodynamics (PK/PD) responses of drugs in vitro [4].
The research endeavors are successfully being commercialized by means of startup companies that have been licensed to develop and market this “Organ-on-a-chip” technology. This technology is being incorporated into routine laboratory research by academic institutions, pharmaceutical companies, cosmetic industry as well as hospitals for the sake of personalized medicine. The technology holds promise to improve the current face of preclinical drug testing by providing an accurate tool for testing the drug efficacy as well as toxicity (Figure 3).
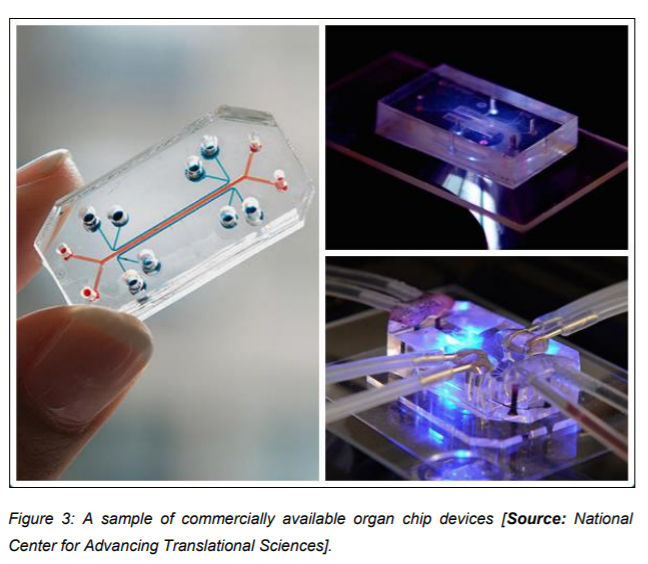
Current and future prospects of the technology
Organ on chip technology has amalgamated wet laboratory biological research with novel computational dry laboratory approaches to facilitate fast
paced and cost effective preclinical drug testing. The approach is currently being employed to identify novel clinical biomarkers, therapeutics, development of vaccines and drug delivery portals. Human stem cells are being incorporated within these chips to further differentiate and develop into specialized cell types and thus pave way for patient specific disease modeling and personalized medicine. A recent development of a 3D-printed
‘Heart Chip’ with soft strain sensors integrated within shows that the technology is gradually progressing towards increased complexity and shall eventually resolve the clinical trial failure dilemma at large and forever.
Author’s Biography
Dr Shripriya Singh Dr. Singh holds a doctorate degree in life sciences from the Indian Institute of Toxicology Research, a premier research center managed by the Council of Scientific and Industrial Research in India. She has research experience of more than five years in the field of Developmental Neurobiology and Neurotoxicology. She is a prolific researcher and writer. She has published a number of peer reviewed research papers in world’s best scientific journals. Dr. Singh has given several talks at national and international meetings in stem cell biology, neuroscience and biotechnology and received first prize for the best oral presentation award at the IBRO/APRC Associate School of Neuroscience: ‘Dawn of the aging world-nipping neurodegeneration in the bud’, held at Selangor, Malaysia from August 8th-14th, 2016. She is an invited reviewer of a number of international research journals and member of many prestigious scientific societies across the globe. Dr. Singh can be reached at shripriyasingh@gmail.com
References and future reading
1. Zhang B & Radisic M (2017) Organ-on-a-chip devices advance to market. Lab on a Chip 17(14):2395-2420.
2. Ergir EE, Bachmann B, Redl HR, Forte G, & Ertl P (2018) Small force, big impact: next generation organ-on-a-chip systems incorporating biomechanical cues. Frontiers in physiology 9:1417.
3. Sun W, et al. (2019) Organ‐on‐a‐ Chip for Cancer and Immune Organs Modeling. Advanced healthcare materials:1801363.
4. https://wyss.harvard.edu/technology/human-organs-on-chip