Photobiomodulation Therapy to Combat Complex Biomedical Problems
Pallavi Tripathi
Intertek Pharmaceutical Services, Hexagon Towers, Crumpsall Vale, M9 8 GQ, Manchester, UK
Abstract
Photobiomodulation (PBM) therapy employs red or near-infrared (NIR) laser. PBM therapy is a rapidly emerging approach that uses a combination of multiple disciplines comprising lasers, high and ultra-high vacuum technologies, biomedical science and engineering and medicine, to name a few. PBM is currently gaining attention for its promise to combat complex medical problems such as cancer, brain disorders, tissue generation etc. Further, PBM technology allows to explore new therapeutic avenues to restore or stimulate healing, reduce pain, increase athletic performance, and improve general wellness. Moreover, recent studies have shown that the ability to stimulate multiple physiological processes can help repair damage caused by injury or disease. In this perspective, we have described some of the notable advances in PBM therapy.
Keywords: Photobiomodulation therapy, brain disorders, burn injuries, skin defects, tissue engineering
DOI: https://doi.org/10.37756/bk.22.4.5.1
Article Type: Perspective
Received: March 20, 2022
Revised: April 19, 2022
Accepted: April 20, 2022
*Corresponding author: Dr. Pallavi Tripathi
E-mail: paltrip@gmail.com
Please cite this article as: Tripathi P, Photobiomodulation Therapy to Combat Complex Biomedical Problems, Biotechnol. kiosk, Vol 4, Issue 5, PP: 1-8 (2022); DOI: https://doi.org/10.37756/bk.22.4.5.1.
Introduction
Photobiomodulation (PBM) therapy is rapidly emerging as a very promising approach to be employed in supportive cancer care, age-related macular degeneration and also battling against Alzheimer’s disease (AD). Additionally, low-dose PBM therapy has been considered to speed up recovery from burns and reduce inflammation. Such low-dose therapy involves a non-ionizing laser source, which is usually placed near or on top of the skin that allows photons to penetrate tissue. This is followed by the interaction with chromophores that are located on cells that results in photophysical and photochemical reactions. Studies have shown that these photon-induced reactions can be leveraged to alter the molecular, cellular, and tissue levels in the body [1].
The cellular and molecular mechanisms of PBM therapy are considered the key aspects for the beneficial effects of PBM. Mainly, the beneficial effects have been suggested to result from the photostimulation of the mitochondrial electron transfer chain (ETC). Previous studies have also shown that application of PBM at optimum fluences (i.e. energy densities) and certain specific wavelengths can results in therapeutic effects that are produced in the target organs without causing any adverse effects. For example, it has been shown that PBM therapy increases cerebral blood flow (CBF), and also augments brain energy metabolism that in turn increases antioxidant defenses [2]. Further, researchers have also demonstrated the ability of PBM to promote neuronal protection and survival. Studies have suggested that such protective mechanisms occur through modulation of anti-apoptotic and pro-apoptotic mediators along with inflammatory signaling molecules along with the stimulation of neurotrophic factors. Moreover, in addition to the beneficial therapeutic effects of PBM at the molecular level, studies have presented evidence of changes occurring also at the behavioral level that include cognitive-enhancement, antidepressant effects and improved sleep [2].
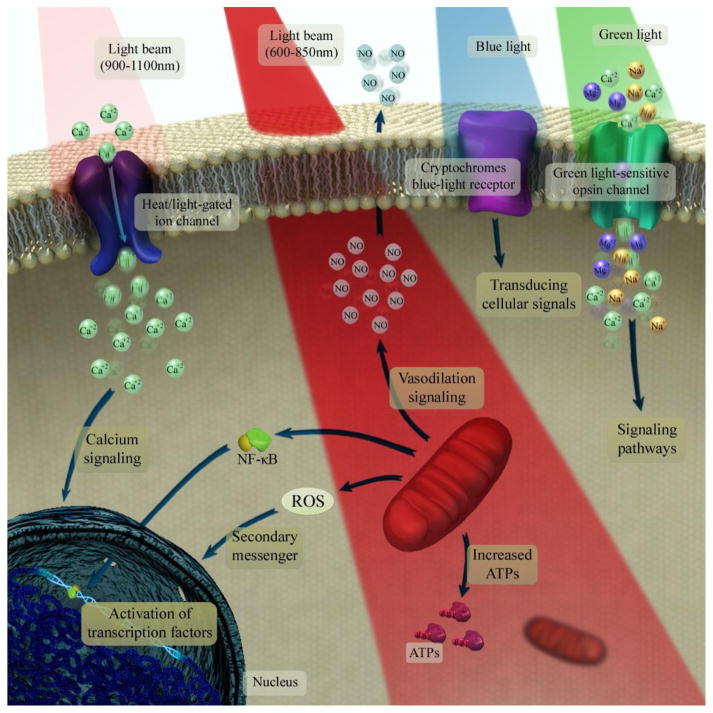
Figure 1: The underlying mechanisms of photobiomodulation at the cellular and molecular levels are depicted. In this process, laser at 600–850 nm is initially absorbed by the mitochondrial electron transfer chain that leads to upregulation of the neuronal respiratory capacity. The NIR laser at range of 900–1100 nm is then absorbed by structured water clusters that are formed in or on a heat/light-gated ion channels. This allows modulation of intracellular Ca2+ levels. Further, the absorption of green laser by neuronal opsin photoreceptors (OPN2–5) can activate transient receptor potential channels. This results in non-selective permeabilization to Ca2+, Na+ and Mg2+. Subsequently, the cryptochromes, which is considered a class of flavoprotein blue-light signaling receptors can absorb blue light. This activates the transducing cellular signals through the optic nerve to the suprachiasmatic nucleus in the brain that regulates the circadian clock [Source: Mol Neurobiol (2018)].
Several studies conducted on PBM (red and NIR spectrum) have shown the resulting photo dissociation of nitric oxide (NO) from the binuclear center (a3/CuB) of cytochrome c oxidase (CCO). Such dissociation of NO has been shown to increase the mitochondrial membrane potential (MMP) as a result of inhibition of NO electron transport in the ETC. This produces an increase in oxygen consumption that ultimately leads to proton gradient and an increase in ATP production [2]. These events lead to the production of reactive oxygen species (ROS) that results in releasing of Ca2+ as versatile second messengers. This subsequently triggers activation of transcription factors and signaling mediators such as NF-κB and resulting in long-lasting effects on cells (Figure 1) [2].
In this perspective, we will describe some notable applications of PBM therapy that have been suggested in some of the most challenging medical problems.
PBM Therapy for Combatting Brain Disorders: Providing Neuroprotection via Anti-Inflammatory and Antioxidant Biological Signaling
Recently, application of red to NIR lights (600–850) for brain PBM therapy has been considered due to the fact that neural tissues contain large amounts of mitochondrial CCO. In some studies, it has been suggested that PBM therapy could be effective for the treatment of a wide range of neurological and psychological disorders that affects various cerebral structures. To this end, studies on clinical brain PBM therapy have focused on conditions such as Alzheimer’s disease ‘AD’, Parkinson’s disease ‘PD’, traumatic brain injury ‘TBI’ and ischemic stroke. In addition to these medical conditions, the application of PBM as a non-invasive modality is also considered in perfectly healthy individuals for the enhancement of their cognitive abilities [2].
Experimental results suggest that irradiation in the wavelength range between 980 nm and 1100 nm can result in different mechanisms of action. This includes stimulation of ion channels and water molecules. Further, it has been shown that PBM that combines with red/NIR lasers could potentially lead to significantly improved cerebral metabolic function, stimulated neurogenesis and synaptogenesis. This could also be beneficial in regulating neurotransmitters, and providing neuroprotection via anti-inflammatory and antioxidant biological signaling that are considered the most important effects of brain PBM therapy (Figure 2) [2]. Extensive preclinical and clinical studies on PBM have been carried out with modest levels of red and NIR lasers. The results have suggested that brain could induce biostimulatory effects without any thermal damage. This could be highly beneficial to improve neurobehavioral deficits associated with many brain disorders that include depression and anxiety and other psychiatric disorders such as schizophrenia, autism, bipolar, attention-deficit hyperactivity and obsessive–compulsive disorders [2].
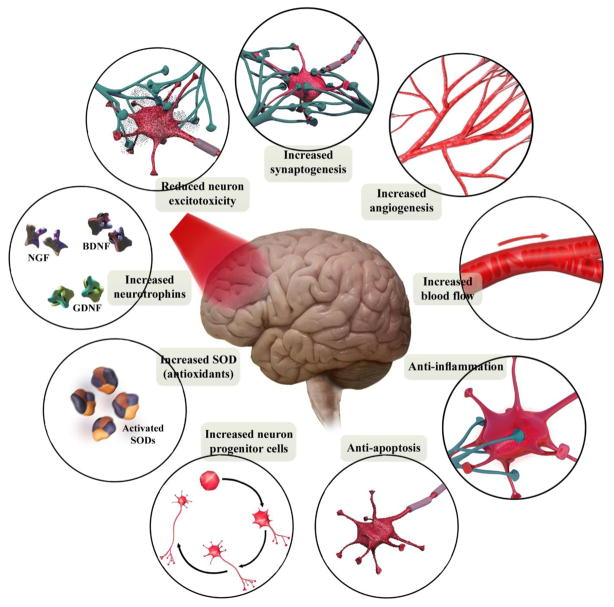
Figure 2: The brain tissue-specific functional processes is schematically illustrated. Brain PBM therapy on a cellular level can reduce apoptosis and excitoxicity while increasing antioxidants, neurotrophins and stimulate neuroprogenitor cells. Whereas, PBM therapy done on a tissue level has been shown to increase blood flow and angiogenesis. This has been shown to result in reducing inflammation and helping neurons to form new connections (BDNF, brain-derived neurotrophic factor; GDNF, glial-derived neurotrophic factor; NGF, nerve growth factor; SOD, superoxide dismutase). [Source: Mol Neurobiol. (2018)].
Healing of Third-Degree Burns: Overcoming Inflammation and Promoting Tissue Generation
Burn injuries are complex medical conditions and it has been estimated that such injuries affect over 6 million people per annum worldwide. Related studies have shown that morbidity involving infections and scarring could result in a high percentage of mortality from burn injuries. To address the issues, clinicians and medical professionals have recommended aggressive clinical management guidelines based on the severity of burns including total body surface area, depth, and co-morbidities. All these procedures and methodologies are based on fundamental burn injury pathophysiology including a range of thermal and cellular stress damage responses along with a prominent inflammatory sequela [3-9].
In a recent study, researchers employed one of the PBM molecular mechanisms of PBM treatments that involved photo activation of latent TGF-β1. The objective was to promote tissue healing and regeneration [9]. They investigated the efficacy of PBM treatments in a full-thickness burn wound healing in C57BL/6 mice. The PBM protocol was optimized by monitoring tissue surface temperature and histology. The dynamic irradiance surface temperature-monitored was then noted. It was reported that PBM protocol improved burn wound healing in mice with elevated TGF-β signaling (phospho-Smad2) while reducing inflammation-associated gene expression (Figure 3) [9]. Further, investigative analysis was conducted on specific contributions of TGF-β1 signaling in the PBM-burn wound healing by using a chimeric TGF-β1/β3 knock-in (TGF-β1Lβ3/Lβ3) mice. An activation of endogenous latent TGF-β1 following PBM treatments was suggested to play a key role in burn wound healing. The new mechanistic insights that were developed in the study could play important roles in improving the safety and efficacy of clinical translation of PBM treatments for tissue healing and regeneration [9].
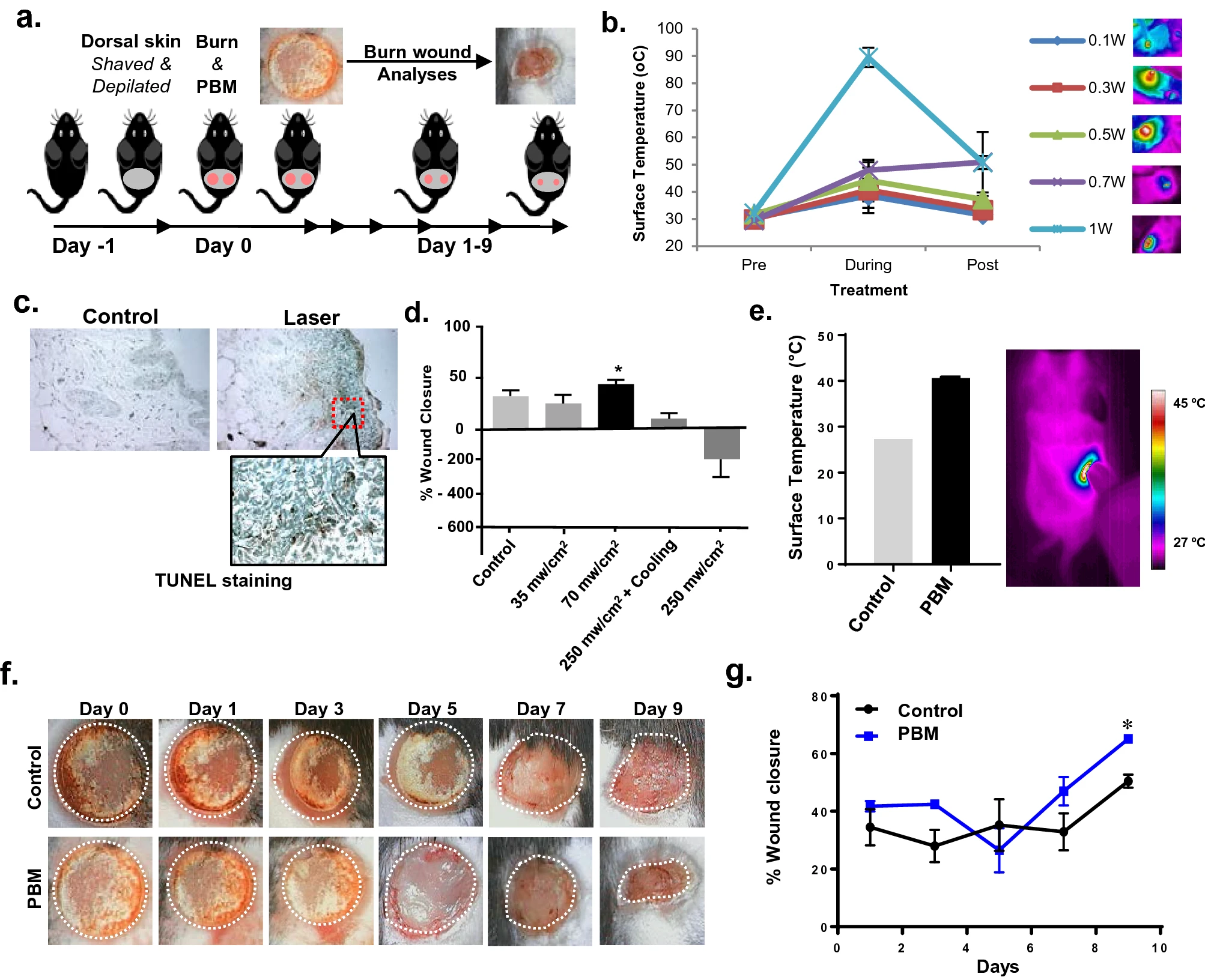
Figure 3: PBM treatment modality is illustrated that improves burn wound healing. (a) A scheme of wound healing experiment is shown that includes photographs of burn wounds obtained every other day up to day 9. (b) Monitoring of tissue surface temperature is conducted during laser treatments with increasing irradiances with a thermal camera. (c) Assessment of tissue damage is done in these tissues with TUNEL staining that indicates phototoxicity at skin temperature above 45 °C. (d) A chart showing burn wound healing following PBM treatments at increasing doses and concomitant surface cooling. (e) Optimal PBM dose treatments are shown that ensure skin surface temperature to be > 45 °C using a dynamic irradiance protocol assessed with thermal imaging (left) and quantitation (right). (f) Photographs of PBM treatments on burn wounds obtained every other day for up to 9 days and compared to untreated controls. (g) Wound areas are digitally quantitated and the results are expressed as means and SDs [Source: Sci. Rep. (2021)].
This study could pave the way for future applications of PBM treatments and the biological rationale for its clinical application in mitigating burn injury and wound healing [9].
Minimizing Distressing Side Effects Resulting from Cancer Therapy
PBM can also be leveraged in the mitigation of numerous distressing side effects that result from an array of different kinds of cancer therapy. Figure 4 (top) shows a summary of the different kinds of cancer-therapy induced side effects that can be addressed by the application of PBM. These side effects have negative consequences on the patient due to the severity that often results in the suspension or discontinuation of the cancer therapy [10].
Recent studies have shown that PBM can have a beneficial effect on cancer. Figure 4 (left) shows the direct effect of the light on the tumor cells themselves. Researchers have considered this approach very promising because it enables overdose that kills the cancer cells (Figure 4). Further, it is known that adenosine triphosphate (ATP) supply is quite limited in cancer cells. It has been thought that the application of PBM may result in ATP boost that may allow the cancer cells to respond to pro-apoptotic cytotoxic stimuli with more efficiently executed cell death that is known as apoptosis. However, such programs are heavily energy dependent. In order words, they require a lot of ATP [10].
Researchers have also proposed a hypothesis that involves normal healthy cells that have an adequate supply of ATP. In healthy cells, the effect of PBM produces a burst of reactive oxygen species (ROS) that could be leveraged induce protective mechanisms and reduce the damaging effects of cancer therapy on healthy tissue (Figure 4, right). It has been also suggested that it could indeed be the case in some anticancer strategies [10].
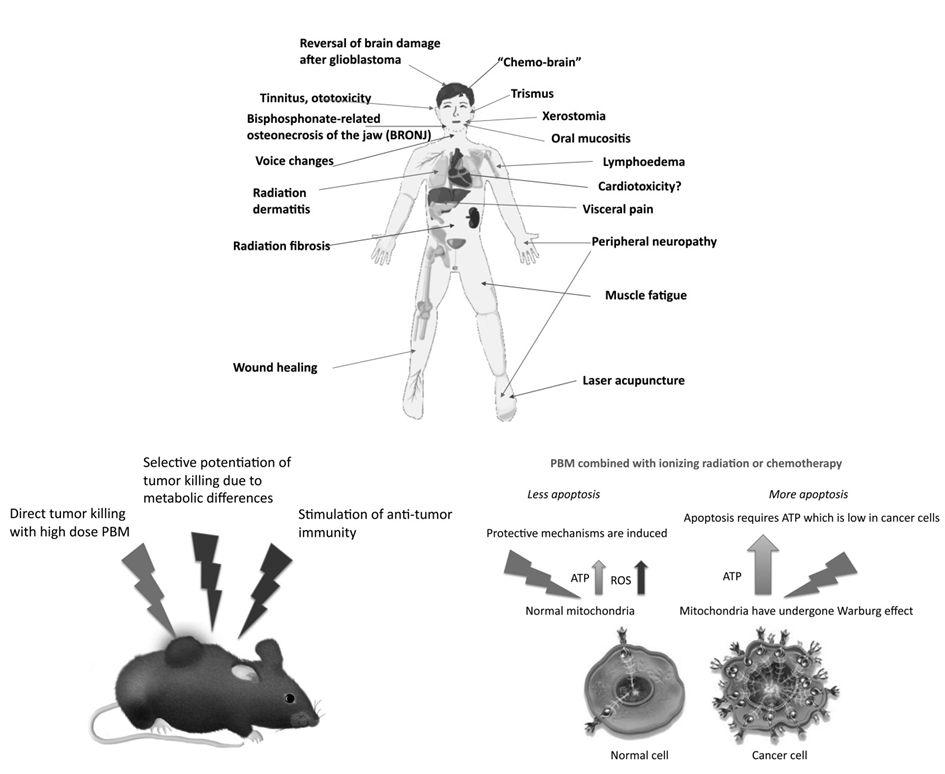
Figure 4: (Top) Schematic depiction of cancer therapy side effects and possible treatment by PBM. (Left) Suggested mechanisms of PBM that could be applied against cancer. (Right) Mechanisms of selective potentiation of cytotoxicity are shown against cancer cells while preserving normal cells [Source: Photomedicine and Laser Surgery (2018)].
Conclusion
Near infrared (NIR) and red lasers assisted photobiomodulation (PBM) therapy has shown a very promising therapeutic pathway for the effective prevention or treatment of complex medical conditions. The field of PBM therapy is rapidly evolving that represents a truly multidisciplinary area of research involving vacuum technology and lasers along with biomedical engineering, medicine and biotechnology. Further developments in PBM therapy in the near future is anticipated that could enable to have new therapeutic avenues for many complex diseases.
References
[1] Lopes Silva RSD, Pessoa DR, Mariano RR, Castro ABS, de Oliveira RA, Ferraresi C, Systematic Review of Photobiomodulation Therapy (PBMT) on the Experimental Calcaneal Tendon Injury in Rats. Photochem Photobiol. 2020, 96(5):981-997, DOI: https://doi.org/10.1111/php.13262.
[2] Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR, Brain Photobiomodulation Therapy: a Narrative Review. Mol Neurobiol 2018, 55(8):6601-6636, DOI: https://doi.org/10.1007/s12035-017-0852-4.
[3] Yadav A, Verma S, Keshri GK & Gupta A, Role of 904 nm superpulsed laser-mediated photobiomodulation on nitroxidative stress and redox homeostasis in burn wound healing. Photodermatol. Photoimmunol. Photomed 2020, 36:208–218, DOI: https://doi.org/10.1111/phpp.12538.
[4] Mosca, RC, Ong AA, Albasha O, Bass K & Arany P, Photobiomodulation therapy for wound care: a potent, noninvasive, photoceutical approach. Adv. Skin Wound Care 2019, 32:157–167, DOI: https://doi.org/10.1097/01.ASW.0000553600.97572.d2.
[5] Keshri GK, Yadav A, Verma S, Kumar B & Gupta A, Effects of pulsed 810 nm Al-Ga-As diode laser on wound healing under immunosuppression: a molecular insight. Lasers Surg Med 2020, 52:424–436, DOI: https://doi.org/10.1002/lsm.23156.
[6] Cancio LC, Salinas J & Kramer GC, Protocolized resuscitation of burn patients. Crit Care Clin 2016, 32:599–610, DOI: https://doi.org/10.1016/j.ccc.2016.06.008.
[7] Gould LJ & May T, The science of hyperbaric oxygen for flaps and grafts. Surg Technol Int 2016, 28:65–72, PMID: 27042776.
[8] Kantak NA, Mistry R, Varon DE & Halvorson EG, Negative pressure wound therapy for burns. Clin Plast Surg 2017, 44:671–677, DOI: https://doi.org/10.1016/j.cps.2017.02.023.
[9] Khan I, Rahman SU, Tang E et al, Accelerated burn wound healing with photobiomodulation therapy involves activation of endogenous latent TGF-β1. Sci Rep 2021, 11:13371, DOI: https://doi.org/10.1038/s41598-021-92650-w.
[10] Hamblin MR, Nelson ST, Strahan JR, Photobiomodulation and Cancer: What Is the Truth? Photomed Laser Surg 2018, 36(5):241-245, DOI: https://doi.org/10.1089/pho.2017.4401.
Rights and Permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third[1]credit line to the permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Creative Commons This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. You are not required to obtain permission to reuse this article. To request permission for a type of use not listed, please contact Biotechnology Kiosk.