

Abstract
The worldwide rapidly increasing amount of plastic waste is considered a major concern in the environmental crisis today. Recycling post-consumer plastics is believed to be a viable approach to mitigate the global plastic waste challenge. Plastic recycling is considered attractive due to the reason that it could help achieve waste valorization while meeting the goals of environmental quality standards. To this end, the current research efforts are heavily focused towards developing innovative technologies for new enzyme discoveries for effective plastic waste management. Recent research has shown biocatalytic depolymerization mediated by enzymes as an efficient and sustainable alternative for plastic treatment and recycling. Using this approach, researchers have discovered different plastic-degrading enzymes that have been derived from microbial sources. Further, the concept of protein engineering has been implemented to modify and optimize plastic-degrading enzymes. In this brief opinion, we describe some of the recent trends and notable advances in mining novel plastic-degrading enzymes through cutting-edge omics-based techniques in order to improve the enzyme catalytic efficiency.
Keywords: biocatalysis; depolymerization; omics-based discovery; enzymes; protein engineering; sustainable plastic recycling
*Corresponding Author
E-mail: saroj1976@gmail.com
- Enzymatic Biocatalysis: Achieving Sustainable Plastic Recycling
There is no doubt that plastic materials have revolutionized the modern world. These materials have tremendously impacted our society. It is said that it would be difficult to imagine our daily lives without plastics. However, there is also a negative aspect of using plastic materials. This flip side of plastics comes from the ever increasing manufacture and extensive use of plastic commodities. Such enormous use of plastics is believed to generate an extraordinary amount of post-consumer plastic waste, which in turn significantly contributes to the global environmental pollution [1]. For example, it is predicted that around 12000 million metric tons of plastic waste could accumulate in landfills and the natural environment by 2050. The consequential effects of this huge accumulation of plastic waste on the global environment could be disastrous as a result of improper handling of plastic waste. This is due to the reason that the debris of plastic waste including microplastics could potentially impose hazardous effects on various organisms that could eventually threaten human health. Another concern is the chemically inert nature of plastics that make them resistant to biodegradation. This further increases their adverse environmental impacts. In view of all these challenges, the current research has focused towards developing innovative technologies for treatment and recycling of post-consumer plastics. This is aimed at achieving both waste valorization and environmental protection. An innovative approach is currently being considered is based on enzymatic biocatalysis. This approach has emerged as an eco-friendly alternative to conventional plastic treatment and recycling methods. Researchers have discovered various microbial plastic-degrading enzymes that are considered promising biocatalyst candidates for plastic depolymerization [1].
However, it is believed that the microbial plastic-degrading enzymes identified so far account only for a small fraction of the enzymes that are relevant to plastic depolymerization in the environment. Hence, researchers have increasingly paid attention to exploring much diverse environments that are helpful to discover new plastic-degrading enzymes with desirable properties and functionalities. This is especially required in view of naturally occurring plastic degrading enzymes that are not implementable for synthetic plastic degradation in industrial applications due to their poor thermostability and low catalytic activity. It is known that synthetic plastic materials that exhibit distinct physical and chemical properties. These properties can make them more resistant to enzymatic attack compared to biogenic polymers. New innovations in omics-based enzyme discovery routes along with protein engineering are being increasingly employed to construct plastic-degrading enzymes with better catalytic efficiency and stability to overcome the existing challenges. Recent research efforts have shown significant promise in omics-based discovering and engineering plastic degrading enzymes to develop a technology platform of enzyme biocatalysis for sustainable plastic treatment and recycling [2-7].
- Plastic-Degrading Enzymes: Omics-Based Discovery Routes
A promising omics approach for mining plastic-degrading enzymes via metagenomics-based methods have recently attracted a lot of research interests. Metagenomics routes have shown huge potential that can be leveraged for the discovery of new enzymes from various ecological habitats [8]. But before considering omics-based approaches, let us first consider the limitations of the conventional culture-dependent method that has been applied to discover most of the implemented plastic-degrading enzymes. It is known that microorganisms expressing the desired enzyme are first enriched and isolated in the culture-dependent method. This occurs under proper cultivation conditions that are followed by strain taxonomical classification. Subsequently, identification of putative enzymes by molecular biological or computational approaches are done (Figure 1A) [1]. However, researchers have shown serious limitations of the culture-dependent method and subsequently the minimum scope of finding new plastic-degrading enzymes. This is due to the reason that only about 1% of the total microorganisms on the planet have been cultured so far. Therefore, the culture-independent metagenomic approach is considered as a more powerful tool that can be exploited for investigating the vast majority of microorganisms from diverse environmental sources [1].
Figure 1B illustrates the overall workflow of metagenomics that is used to discover plastic-degrading enzymes. It has been shown that choosing appropriate screening methods is critically important for an efficient metagenomic mining [1]. Resarchers have used two common methods to screen the metagenomic library. These include sequence-based screening and function-based screening. In sequence-based screening, sequence similarity comparison and functional gene annotation are done by searching bioinformatic databases. A typical example is a poly(ethylene terephthalate) (PET) hydrolytic enzyme (PET2). This enzyme was discovered through in-silico sequence based screening from metagenome databases. This was achieved by using a search algorithm powered by a hidden Markov model [1]. Lately, researchers have paid attention to a number of gene sequences that are similar to the ones encoding known enzymes. These enzymes that are known to have activity to degrade polyurethane (PU) plastics are retrieved from landfill derived metagenomes. In-silico sequence-based metagenomic screening is considered relatively rapid and a cost-effective process for enzyme mining. There are some limitations of this process. For example, the metagenomics process has been shown to be limited by the size and gene annotation quality of current databases of known plastic-degrading enzymes. In addition, it has been shown that metagenomics process could potentially exclude new families of plastic-degrading enzymes with low sequence similarity to previously characterized enzymes. Further, another limitation lies in the fact that sequence similarities do not guarantee plastic-degrading activity. To overcome these limitations, more characterization along with validation of enzyme functionality has been recommended [8-14].
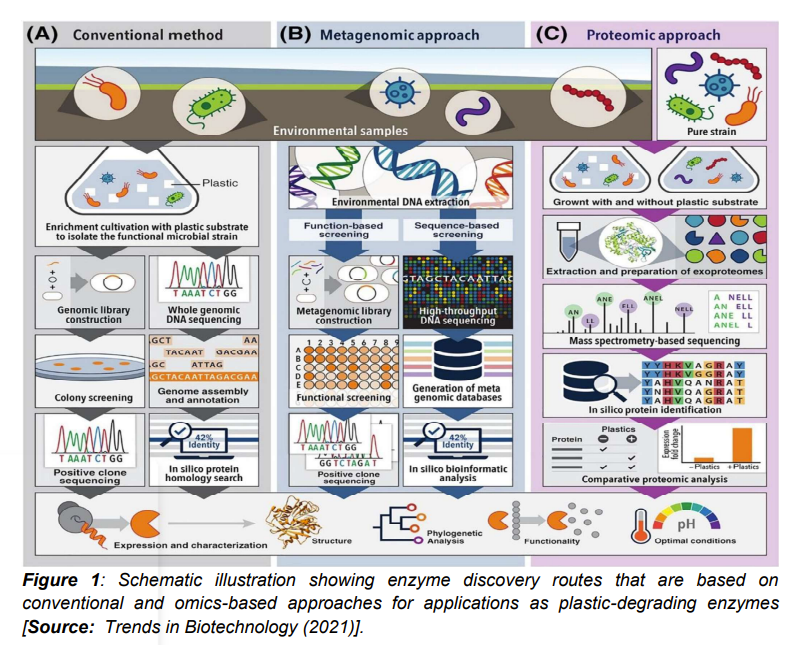
On the other hand, researchers have shown huge potential of another omics-based route that involves proteomics-based approach. It is believed that proteomics-based approach can be leveraged for direct detection and quantification of protein expression. To this end, proteomics-based approach has proven to be very promising in mining new enzymes from a broad range of microbial sources for new biotechnological applications [1, 15]. The commonly used workflow is shown in Figure 1C for the proteomic approach for mining plastic-degrading enzymes [1]. In this process, the first step involves growth of pure or environmental microbial consortia with and without the plastic substrate. The process without plastic substrate is chosen because the presence of plastics is believed to differentially induce the functional microorganisms to express enzymes with plastic hydrolytic activity. Subsequently, extraction and digestion of proteins take place. Microbial cultures are subjected to sequencing that are followed by protein identification via bioinformatic analysis. Studies have suggested that the principal target is usually exoproteome at the time of screening for potential plastic-degrading enzymes. This is attributed to the insoluble synthetic plastics that are unable to enter the microbial cell. In addition, enzymes that are engaged in depolymerization are usually secreted extracellularly. In most of the studies, researchers have demonstrated the effectiveness of the proteomic technique in identifying various enzymes involved in plant biopolymer degradation. This has inspired the implementation of proteomics-based approach in the discovery of novel plastic-degrading enzymes [15-17].
In the process of mining plastic-degrading enzymes, comparative proteomics is usually employed. This process is based on certain presumption that incubation with plastics would stimulate the expression of enzymes that are involved in plastic depolymerization [1]. Using this assumption, researchers identified several novel putative polyesterases that are involved in PBAT degradation. This was achieved by comparative analysis of the exoproteome of the bacterium Pseudomonas pseudoalcaligenes and fungus Knufia chersonesos. This demonstrated the effectiveness of the method in mining plastic-degrading enzymes that are especially suitable for microorganisms with unavailable annotated genomic data. In a related study, researchers produced a polyhydroxybutyrate (PHB) depolymerase ALC24_4107 by Alcanivorax sp. 24 with activity in hydrolyzing. This showed a variety of natural and synthetic polyesters that was discovered via the comparative exoproteomic approach [18-20].
Despite notable advances, the field of proteomics-guided discovery of plastic-degrading enzymes is an evolving area of research and development. Most of the studies were conducted with pure microbial cultures. It is still considered a difficult process for high-quality protein extraction and limited availability of databases for downstream bioinformatic analysis. Therefore, it is considered a challenge to achieve direct identification of plastic-degrading enzymes through metaproteomics from complex environmental samples. To overcome the challenges, researchers have considered protein engineering of plastic-degrading enzyme. It is believed that protein engineering techniques can be leveraged to improve the catalytic performance of plastic-degrading enzymes. To this end, researchers have paid attention to two categories of approaches in protein engineering that involve rational design and directed evolution. In the rational design approach, the protein of interest is modified based on the knowledge of protein structure and mechanistic characteristics, computational simulation as well as modeling. There are several reports on engineering plastic-degrading enzymes that have been shown to utilize rational design due to the available structural and mechanistic information for many of these enzymes [1]. With respect to the directed evolution based approach, it is usually believed that the lack of efficient high-throughput screening techniques is a main barrier for this approach to be employed for plastic-degrading enzymes. There is a single report that has employed direct evolution to engineer PHB depolymerase from Ralstonia pickettii T1. However, this method was shown not being able to acquire any variant with improved activity [21].
- Concluding Remarks & Future Perspective
There are innovative recent research efforts that have been reported on enzyme biocatalysis. These reports have shown a promising route based on a green chemistry alternative for sustainable plastic waste management and recycling. Especially, it is believed that the enzyme-mediated biocatalytic degradation could pave the way for future breakthroughs involving integration into the plastic recycling process. This could be utilized either to cooperate with or replace current chemical recycling based processes. These routes have shown the possibility that with some mechanical pretreatment, the plastic materials could be transferred into bioreactors containing plastic-degrading enzymes for biocatalytic depolymerization. Subsequently, it is believed that the produced chemical molecules could either be employed as building block monomers to synthesize new plastic products involving a closed-loop recycling method or as feedstocks that could be used for conversion into high-value chemicals in an open-loop upcycling method. Particularly, researchers are hopeful with the rapid advances in omics based techniques together with synthetic biology, and advances in protein engineering that together offer a wide array of powerful toolkits. These toolkits are believed to play key roles in the future studies to discover, characterize, and modify plastic-degrading enzymes. This is in turn thought to pave the way to opening up new possibilities to acquire novel biocatalysts with innovative properties for efficient and cost-effective plastic depolymerization. These efforts are thought to lead to novel solutions to the critical challenges that are still remaining for transitioning enzyme biocatalysis for plastic recycling to the industrial level applications in future.
References
[1] Zhu, B.; Wang, D.; Wei, Na. Enzyme discovery and engineering for sustainable plastic recycling. Trends in Biotechnology 2021, 40, 22-37, doi: https://doi.org/10.1016/j.tibtech.2021.02.008.
[2] Redondo-Hasselerharm, P. E. et al. Nano- and microplastics affect the composition of freshwater benthic communities in the long term. Sci. Adv 2020, 6eaay4054, doi: https://doi.org/10.1126/sciadv.aay4054.
[3] Koelmans A. A. et al. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 2019, 155, 410-422, doi: https://doi.org/10.1016/j.watres.2019.02.054.
[4] Seeley M.E. et al. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372, doi: https://doi.org/10.1038/s41467-020-16235-3
[5] Boots B. et al. Effects of microplastics in soil ecosystems: above and below ground. Environ. Sci. Technol. 2019, 53, 11496-11506, doi: https://doi.org/10.1021/acs.est.9b03304.
[6] Chamas A. et al. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494-3511, doi: https://doi.org/10.1021/acssuschemeng.9b06635.
[7] Wei R. et al. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 2020, 3, 867-871, doi: https://doi.org/10.1038/s41929-020-00521-w.
[8] Ufarte L. et al. Metagenomics for the discovery of pollutant degrading enzymes. Biotechnol. Adv. 2015, 33, 1845-1854, doi: https://doi.org/10.1016/j.biotechadv.2015.10.009.
[9] Taniguchi I. et al. Biodegradation of PET: current status and application aspects. ACS Catal. 2019, 9, 4089-4105, doi: https://doi.org/10.1021/acscatal.8b05171.
[10] Yoshida S. et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science. 2016, 351, 1196-1199,
doi: https://doi.org/10.1126/science.aad6359.
[11] Sankara Subramanian S.H. et al. RemeDB: tool for rapid prediction of enzymes involved in bioremediation from high-throughput metagenome data sets. J. Comput. Biol. 2020, 27, 1020-1029, doi: https://doi.org/10.1089/cmb.2019.0345.
[12] Danso D. et.al. New insights into the function and global distribution of polyethylene terephthalate (PET)-degrading bacteria and enzymes in marine and terrestrial metagenomes. Appl. Environ. Microbiol. 2018, 84: e02773-e02817, doi: https://doi.org/10.1128/AEM.02773-17.
[13] Gaytan I. et al. Degradation of recalcitrant polyurethane and xenobiotic additives by a selected landfill microbial community and its biodegradative potential revealed by proximity lgation-based metagenomic analysis. Front. Microbiol. 2019, 10, 2986, doi: https://doi.org/10.3389/fmicb.2019.02986.
[14] Urbanek A.K. et al. Biochemical properties and biotechnological applications of microbial enzymes involved in the degradation of polyester-type plastics. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868140315, doi: https://doi.org/10.1016/j.bbapap.2019.140315.
[15] Bers K. et al. A novel hydrolase identified by genomic-proteomic analysis of phenylurea herbicide mineralization by Variovorax sp. strain SRS16. Appl. Environ. Microbiol. 2011, 77, 8754-8764, doi: https://doi.org/10.1128/AEM.06162-11.
[16] Sturmberger L. et al. Synergism of proteomics and mRNA sequencing for enzyme discovery. J. Biotechnol. 2016, 235, 132-138, doi: https://doi.org/10.1016/j.jbiotec.2015.12.015.
[17] Tesei D. et al. Shotgun proteomics reveals putative polyesterases in the secretome of the rock-inhabiting fungus Knufia chersonesos. Sci. Rep. 2020, 10, 9770, doi: https://doi.org/10.1038/s41598-020-66256-7.
[18] Zadjelovic V. et al. Beyond oil degradation: enzymatic potential of Alcanivorax to degrade natural and synthetic polyesters. Environ. Microbiol. 2020, 22, 1356-1369, doi: https://doi.org/10.1111/1462-2920.14947.
[19] Wallace P.W. et al. PpEst is a novel PBAT degrading polyesterase identified by proteomic screening of Pseudomonas pseudoalcaligenes. Appl. Microbiol. Biotechnol. 2017, 101, 2291-2303, doi: https://doi.org/10.1007/s00253-016-7992-8.
[20] Biswas R. Sarkar A. ‘Omics’ tools in soil microbiology: the state of the art. in: Adhya T.K. Advances in Soil Microbiology: Recent Trends and Future Prospects. Springer Singapore, 2018, 35-64, ISBN: 978-981-10-6178-3.
[21] L.T. Tan, et al. Directed evolution of poly[(R)-3-hydroxybutyrate] depolymerase using cell surface display system: functional importance of asparagine at position 285, Appl. Microbiol. Biotechnol., 2013, 97, 4859-4871, doi: https://doi.org/10.1007/s00253-012-4366-8.