Sensory science
August 30, 2020Abstract1 vol2 issue7
August 30, 2020
Abstract
Cellular senescence is believed to possess a clear hallmark of normal chronological aging. The current research focus has been to better our understanding of the fundamental causes of aging and identification of its potential therapeutic targets as well as modulators of senescence cells. This is considered of paramount importance in maintaining a lasting health and longevity. Senolytics correspond to the drug-mediated process that seek to develop modulating agents for senescence cells to delay, prevent, alleviate, or reverse age-related diseases. Here, we present some of the recent notable breakthroughs in regulating cellular senescence for a number of significant anti-aging and pro-health therapeutic applications of senolytics.
*E-Mail: shyabiswas@biotechkiosk.com
1. Introduction: Regulating Senescent Cells
Senescent cells are believed to accumulate with age in multiple tissues that may cause age‐associated disease and functional decline. Genetic or drug‐mediated specific ablation of senescent cells has been considered to ameliorate a wide range of age‐associated disabilities and diseases in mice [1-3]. The drug-mediated process is often referred to as senolytics, which seek to develop agents to delay, prevent, alleviate, or reverse age-related diseases. To this end, early research in the field demonstrated the possibility of clearing senescent cells by activating a drug-inducible ‘suicide’ gene that was shown to enhance health span and delay multiple age-related phenotypes in genetically modified progeroid mice [3]. It is believed that senolytics based therapeutic interventions could reduce the burden of senescent cells. This could potentially ameliorate age-related disabilities and chronic diseases as a group [4].
Thus, in view of the huge medical benefits that are associated with senolytics, current industrial research and developments indicate that a large number of pharmaceutical companies are now actively engaged in the discovery of senolytic drugs that can target senescent cells [5]. However, it is also believed that many drugs including antibiotics that are approved by the US Federal Drug and Administration (FDA) may also possess senolytic activity and by employing these drugs would dramatically accelerate the clinical use of the senolytic drugs in any anti-aging drug trials [5]. A number of research groups have studied several candidate senolytic drugs in-vitro [4]. Among most of the senolytic agents that have been tested, Dasatinib (D) and Quercetin (Q) have been shown to be particularly promising in clearing senescent cells. Further, the combination of D+Q has been considered very effective that can selectively target a broader range of senescent cell types. It was reported that in both senescent and nonsenescent cultured preadipocytes, D and Q reduced expression of the anti-apoptotic regulator PAI-2 [4].
2. Targeting Senescent Cells with Clinically-Approved Drugs
In a recent research, a simple but innovative senolytic screening approach was shown that allowed systematically identification of clinically-approved drugs (Figure 1) to target the senescence phenotype of human fibroblasts [5]. To achieve the goals, MCR-5 and BJ cells were treated with BrdU (a DNA-damaging agent). By employing this screening approach, two clinically-approved macrolide antibodies (namely, Azithromycin and Roxithromycin) were demonstrated to preferentially exhibit senolytic activity [5].
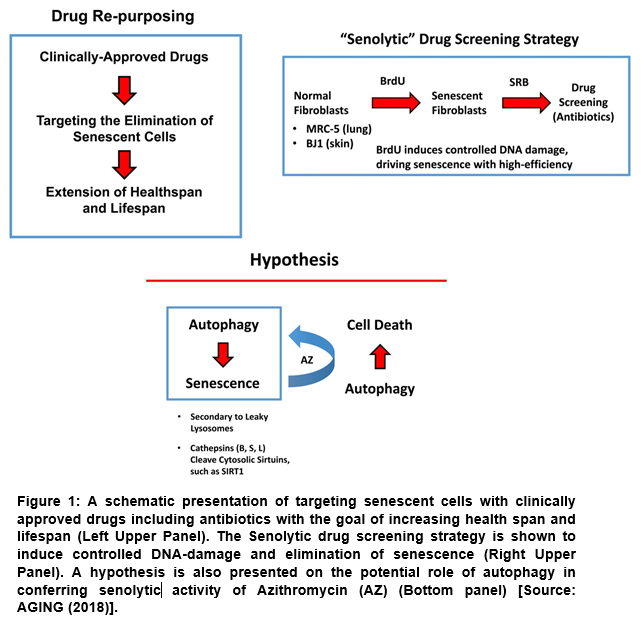
In this study, the methodology used a simplified screening assay that enabled identification and repurposing of clinically-approved therapeutics with senolytic activity for the treatment of aging and aging-associated disorders (Figure 1) [5]. Subsequently, two independent normal and non-immortalized human fibroblast cell lines, MCR-5 (for screening) and BJ (for validation) derived from human lung and skin tissues were employed. The responses of normal fibroblasts and senescent fibroblasts were then directly compared, and drugs that preferentially killed senescent fibroblasts were considered the senolytic drugs. This approach enabled identification of two erythromycin-family members, Azithromycin and Roxithromycin that preferentially targeted senescent fibroblasts (Table 1). It was observed that Azithromycin preferentially targeted and removed almost 97% senescent cells with great efficiency (Table 1) [5]. This represented a near 25-fold reduction in senescent cells. However, Erythromycin was found ineffective in any senolytic actions [5].
The hypothesis behind this study (Figure 1) was based on the previous experimental observations of activation of autophagy by inhibition of mTORC that was shown to efficiently suppress senescence phenotypes [6]. It is known that autophagic cells have greater likelihood of becoming senescent. This process is known as autophagy-senescence transition (AST) [5]. It has been shown that cells accumulate auto-phagic organelles (lysosomes and auto-phagosomes) during autophagy. These structures are believed to involve sequestering of dangerous proteases, including the cathepsins (B, S and L) [5]. Further, once the cathepsins are in the cytosol, they can proteolytically cleave the sirtuins, such as SIRT1 that can drive the senescence pheno-type [7]. In this study, Azithromycin (AZ), which is a weak autophagy inducer is found to preferentially target senescent cells [5]. Thus, the authors hypothesized that the induction of autophagy in senescent cells can drive cell death (Figure 1) [5].
Table 1: The effects of antibiotics on BrdU-treated senescent MRC-5 fibroblasts. The biological effects of three antibiotics, namely Erythromycin, Azithromycin and Roxithromycin, on cell viability are summarized. Erythromycin was found to be completely ineffective. Roxithromycin and Azithromycin had no effect at a lower dose (50 µM), but selectively eliminated large numbers of senescent cells at 100 µM. Azithromycin was particularly found to be the most selective compound, as it eliminated senescent cells without affecting control cells [Source: AGING (2018)].
3. Human Clinical Trial of Senolytics in Chronic Health Dysfunctions
In a more recent research on senolytics in chronic health dysfunctions, researchers reported a first-in-human clinical trial of Dasatinib (D) plus Quercetin (Q) in individuals with diabetic kidney disease [8, 9]. The combination of D+Q was shown to be quite effective in selectively eliminating senescent cells. The combined senolytics power of D+Q results in transiently disabling pro-survival networks that defend these cells against their own apoptotic environment [8]. This apparently first clinical trial of D+Q senolytics exhibited improved physical function in patients with pulmonary fibrosis (IPF), which is a fatal senescence induced disease [8]. This clinical trial suggests that D + Q therapy-induced alleviation of tissue dysfunction and disease progression could potentially benefit complex medical condition such as diabetes and chronic kidney disease (CKD) in humans [8]. Administration of D + Q demonstrated significantly reduced (p = 0·001) adipose tissue p16INK4A+ cells, where raw values were reduced by 35% (Figure 2a). Further, senolytics caused a significant reduction (p = 0·009) in adipose tissue p21CIP1+ cells as well, with raw values being decreased by 17% (Figure 2b) [8]. In addition, D + Q also reduced (p = 0·005) SAβgal+ cells in which raw values were observed to be reduced by 62% (Figure 2c) [8].
This human clinical trial shows the senolytics promise of D + Q in decreasing senescent cells that gives the first direct evidence that senolytics are effective in humans [8]. All these preliminary examples of the power of senolytics in anti-ageing therapy show enormous promise to regulate senescence cells and increase healthy lifespans in humans. The first clinical trial of senolytics in human in combating chronic health dysfunctions and age-related diseases gives significant boost to the concept of senolytics based future biomedicine [9].
4. Conclusion and Outlook
In general, cellular senescence has been recognized as a hallmark of aging. The consensus is that the deficient clearance of senescent cells by the immune system in aged tissues results in accumulation of senescent cells that subsequently exert deleterious effects and eventually jeopardize tissue homeostasis. The therapeutic perspectives offer a whole host of new opportunities to develop anti-ageing therapies along with therapeutics to battle age-related complex diseases.
The field of senolytics is a relative novel and unexplored research area. Senolytic agents are being investigated for their clinical relevance. We anticipate a rapid growth in the field of senolytics in the near future including clinical trials. It is possible to identify pre-existing clinically-approved antibiotics with senolytic activity, for drug repurposing as anti-aging drugs that can be used to target senescent fibroblasts and other age-related medical conditions. In addition to senolytics, novel methodologies for the detection of cellular senescence and new technologies applied to the analysis of mitochondrial biochemistry are expected to be developed.
References
[1] Baker, D.J., Childs, B.G., Durik, M., Wijers, M.E., Sieben, C.J., Zhong, J., et al., 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530(7589):184-9.
[2] Baar M. P., Brandt R. M., Putavet D. A., Klein J. D., Derks K. W., Bourgeois B. R., De Keizer P. L. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell, 169, 132–147 (2017).
[3] Baker D. J., Wijshake T., Tchkonia T., LeBrasseur N. K., Childs B. G., et. al., Clearance of p16Ink4a‐positive senescent cells delays ageing‐associated disorders. Nature, 479, 232–236 (2011).
[4] Yi Zhu, Tamara Tchkonia, Tamar Pirtskhalava, Adam C Gower, Husheng Ding, Nino Giorgadze et. al., The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs, Aging Cell 14, pp644–658 (2015).
[5] Bela Ozsvari, John R. Nuttall, Federica Sotgia, Michael P. Lisanti, Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts, Aging (Albany NY), (11): 3294–3307 (2018), DOI:
[6] Correia-Melo C et al Rapamycin improves healthspan but not inflammaging in nfkappab1(-/-) mice. Aging Cell 18(1):e12882 (2018), DOI:
[7] Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol.; 180:973–83 (2012), DOI:
[8] LaTonya J. Hickson, Larissa G.P.Langhi Prata, Shane A. Bobart, Tamara K. Evans, Nino Giorgadze et. al., Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease, EBioMedicine, 47, 446-456 (2019), DOI:
[9] Jamie N. Justice, N. Justice, Anoop M. Nambiar, Tamar Tchkonia, Nathan K. LeBrasseur, Rodolfo Pascual, Shahrukh K. Hashmi, Larissa Prata, Michal M. Masternak, Stephen B. Kritchevsky, Nicolas Musi, James L. Kirkland, Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study, EBioMedicine, 40, 554-563 (2019), DOI: