Structural Preservation and Analysis of Biological Macromolecules in Cryogenic Microscopy for Future Drug Discovery
Megha Agrawal
Betterhumans Inc. 3653 NE 77th Ave, Gainesville, FL 32609, USA
USA Prime Biotech LLC, 1130 NW 6th Street, Suite A-2, Gainesville, FL 32601, USA
Abstract
Lately, cryogenic electron microscopy (cryo-EM) has emerged as a cutting-edge biophysical technique for structural analysis of biological macromolecules and assemblies. In structural analysis, structural preservation of samples such as isolated cell organelles, viruses, bacteria, eukaryotic cells and other samples for soft-matter applications is extremely important. Due to the advantage of cryo-EM technique to preserve the structures of biological macromolecules, there is a tremendous research interest in Cryo-EM among biotechnologists, medical professionals and biomaterials scientists that seek an unaltered view on the architecture of a vast array of clinically important biological specimens. In this perspective, we have described a brief overview of the recent progress in this field.
Keywords: Cryo-EM, marcomolecules, structural preservation, drug discovery
DOI: https://doi.org/10.37756/bk.22.4.6.1
Article Type: Perspective
Received: May 5, 2022
Revised: May 19, 2022
Accepted: May 20, 2022
*Corresponding author: Dr. Megha Agrawal
E-mail: meghaagra@gmail.com
Please cite this article as: Agrawal M, Structural Preservation and Analysis of Biological Macromolecules in Cryogenic Microscopy for Future Drug Discovery, Biotechnol. kiosk, Vol 4, Issue 6, PP: 1-8 (2022); DOI: https://doi.org/10.37756/bk.22.4.6.1
Introduction
For cryo-electron microscopy of biological samples, it is critical to structurally preserve biological samples when they are used under cryogenic conditions below their recrystallization temperature. The goal is to preserve the native state until imaging is accomplished. In addition, avoidance of contamination is required during sample transfer and handling. Despite advances made in this field, it is still a challenge to conserve the pristine architecture of frozen-hydrated biological samples [1-3].
Researchers have shown vitrification of the sample within milliseconds as a viable option to prevent the physical arresting of the sample in a frozen-hydrated state. This process helps result in a sample that is embedded in amorphous ice. This subsequently helps avoiding any structural changes due to ice crystal growth. However, handling of vitrified samples is proven to be a delicate process. Further, another challenge is the imaging of the frozen-hydrated state that requires the temperature of the specimen to be maintained below the glass transition temperature of water, 138 K (−135°C). Further, the unprotected sample could also pose a challenge as it could act as a cold trap that subsequently could be susceptible to any kind of contamination. The other challenge in the samples preparation is the problem that arises from dehydrated samples. This is due to the fact that samples turn hygroscopic and therefore they are required to be transported in an anhydrous environment [1].
New Approach for Transferring Frozen-Hydrated Samples for Cryo-EM
Researchers have addressed these challenges involving frozen-hydrated biological sample preparation and transfer for cryo-EM imaging. To this end, high-vacuum technology based sample transfer process has been demonstrated for a seamless sample transfer along with preserving the native state of the sample. A promising approach relies on the handling of the sample in liquid nitrogen (lN2). This can be done in a dry inert gas atmosphere or in a clean vacuum environment under controlled temperature conditions at all times [1]. It is to be noted that most of the modern electron microscopes that are equipped with storage devices and directly connected to the column allow samples to be stored and transferred to the microscope stage. This is done under high-vacuum environment at cryogenic conditions. However, one problem is still faced with the high-vacuum conditions that have to be broken when transferring to other devices for further processing or analysis [1].
To overcome this challenge, recent advances in high-vacuum cryogenic transfer technology have focused on developing a high-vacuum cryo-transfer system that can streamline the entire handling of frozen-hydrated samples from the vitrification process to low temperature imaging for scanning transmission electron microscopy and transmission electron microscopy [1]. Especially, a high-vacuum cryo-transfer system has been shown that overcomes the challenges and limitations of the existing systems. This high-vacuum transfer assembly was designed to streamline the handling of frozen-hydrated samples. This included a high turnover of specimens per working day coupled with a safe storage capacity for prepared samples. The advantages include components such as sample cartridge, storage device and high-vacuum cryo-shuttle (HVCS) (Figure 1) [1]. The performance of the developed cryogenic transfer system for frozen-hydrated samples was shown by employing an in-lens cryo-scanning transmission electron microscope. Tobacco Mosaic virus (TMV) and amorphous carbon foil. Subsequently, the mass of the TMV along with the thickness of the carbon film were measured for comparison before and after the transfer sample process. This eliminated the possibility of contamination that would falsify the scattering characteristics of the sample material [1].
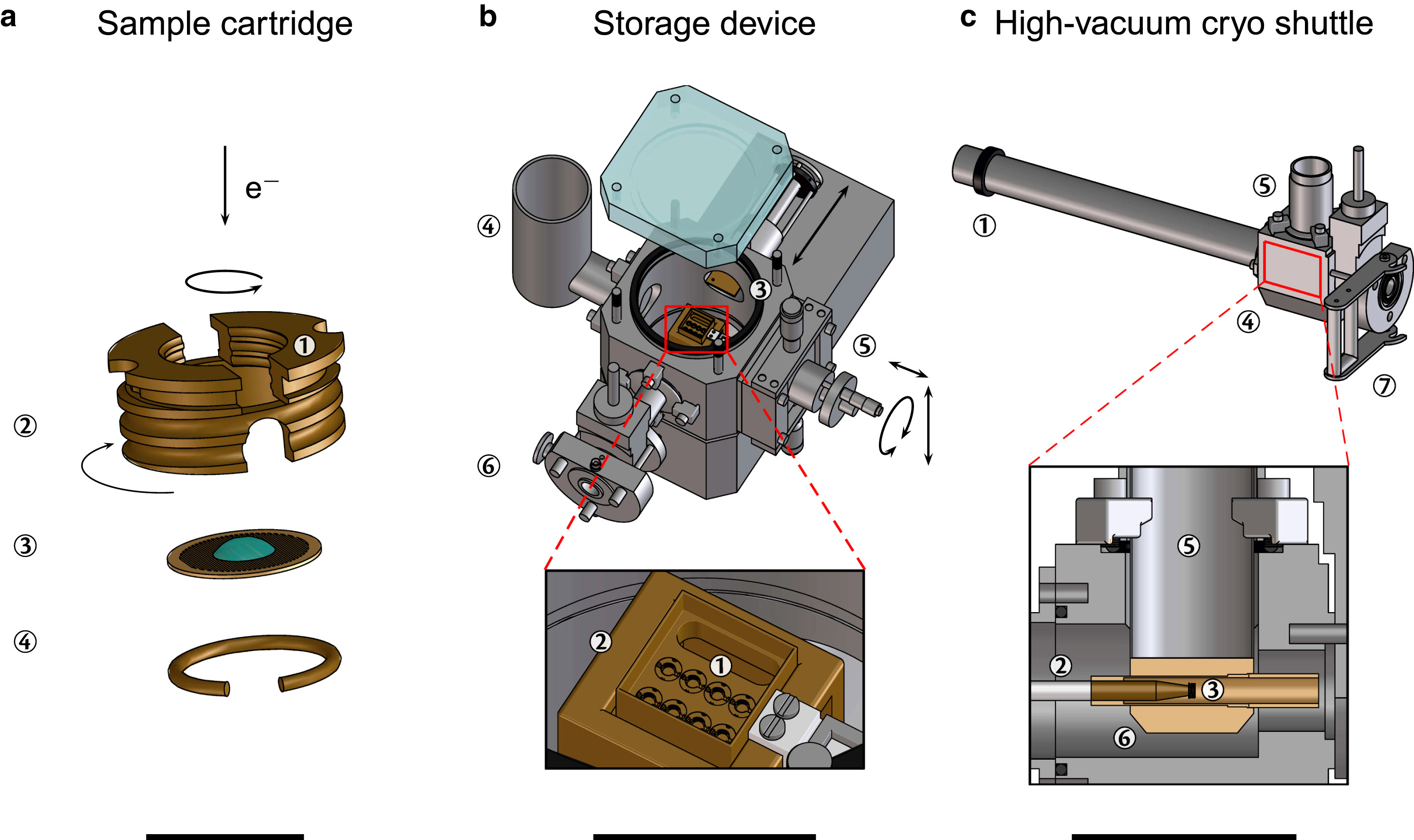
Figure 1 (a-c) Schematic depiction of the high-vacuum cryo-transfer system and storage device that includes up to eight cartridges that can be mounted in the transport box along with a high-vacuum cryo-shuttle (HVCS) [Source: Biophysical Journal (2016)].
As Figure 1 shows, the operation of the system is based on a magnetically linked shaft (point 1) of the HVCS that is moved inside the chamber of the storage device for dismounting the cartridge. The front of the precooled shaft (point 2) is then screwed to the cartridge (point 3). The cartridge can subsequently be unscrewed from the transport box and inserted into the chamber of the HVCS (point 4) after this gets attached [1]. To make the temperature to remain well below the recrystallization temperature of −135°C, the role of magnet is important that enables the cartridge to be connected to the wall of the Dewar-vessel of the HVCS (point 5) once the shaft gets retracted [1]. Further, the anti-contaminator and the high-vacuum environment is represented by point 6 that helps achieve a contamination and artifact free transfer of frozen-hydrated samples. The HVCS is finally then docked onto the counterpart of the docking-station (point 7) of the target device [1].
Studying Biomolecular Complexes
In a recent study, cryo-EM was employed for structure determination of isolated biomolecular complexes across a wide molecular mass range including proteins with several tens kilo-Daltons to virus particles with many mega-Daltons and to whole cells (Figure 2) [4]. Several advantages of cryo-EM was shown over the standard structural characterization techniques such as X-ray crystallography and NMR spectroscopy. These include a much smaller amount of sample and flexibility of a larger variation of specimen types such as single protein molecules, large protein complexes, thin-protein crystals, virus particles, helical fiber complexes, bacteria, cells and even entire tissue sections, to name a few [4]. It also has a limitation related to the maximum observable object size, which is the specimen thickness that can be penetrated with the electron beam. Further, it has been shown that observation areas in the case of eukaryotic cells could be limited to the periphery of the cell body. However, one can thin the sample before observation by cryo-sectioning or FIB-milling [4].
In other studies, a particular challenge has been addressed in cryoEM that poses a limitation in investigating samples with lower molecular weight. Researchers showed ~2.8 Å and ~3.2 Å structures of methemoglobin, which demonstrated that distinct conformational states can be identified within a dataset for proteins as small as 64 kDa. Furthermore, it provided the sub-nanometer cryo-EM structure of a sub-50 kDa protein [5]. The image contrast was generated with the specimen-tolerated electron dose that restricted minimum detectable size of isolated biomolecules. The permissible electron dose was limited by radiation damage to the specimen that depended on acceleration voltage and spatial resolution as well as sample properties. For example, less than 20 e−/Å2 at 200 kV accelerating voltage was used to achieve near-atomic resolution [4].
Studies have shown that the lower limit of molecular weight accessible by cryo-EM can be extended towards the upper molecular weight range of NMR, which is about 50 kDa [4]. To this end, the structure of isocitrate dehydrogenase 1 (IDH1; 93-kDa) was shown by single particle cryo-EM at 3.8 Å resolution (Figure 2) [4]. The low signal-to-noise ratio (SNR) was attributed to the small mass giving rise to electron scattering. With respect to large proteins, the structure of the 64-kDa human hemoglobin particle was analyzed at 3.2 Å resolution by contrast-enhanced cryo-EM using a Volta phase plate (VPP) (Figure 2) [4, 6].
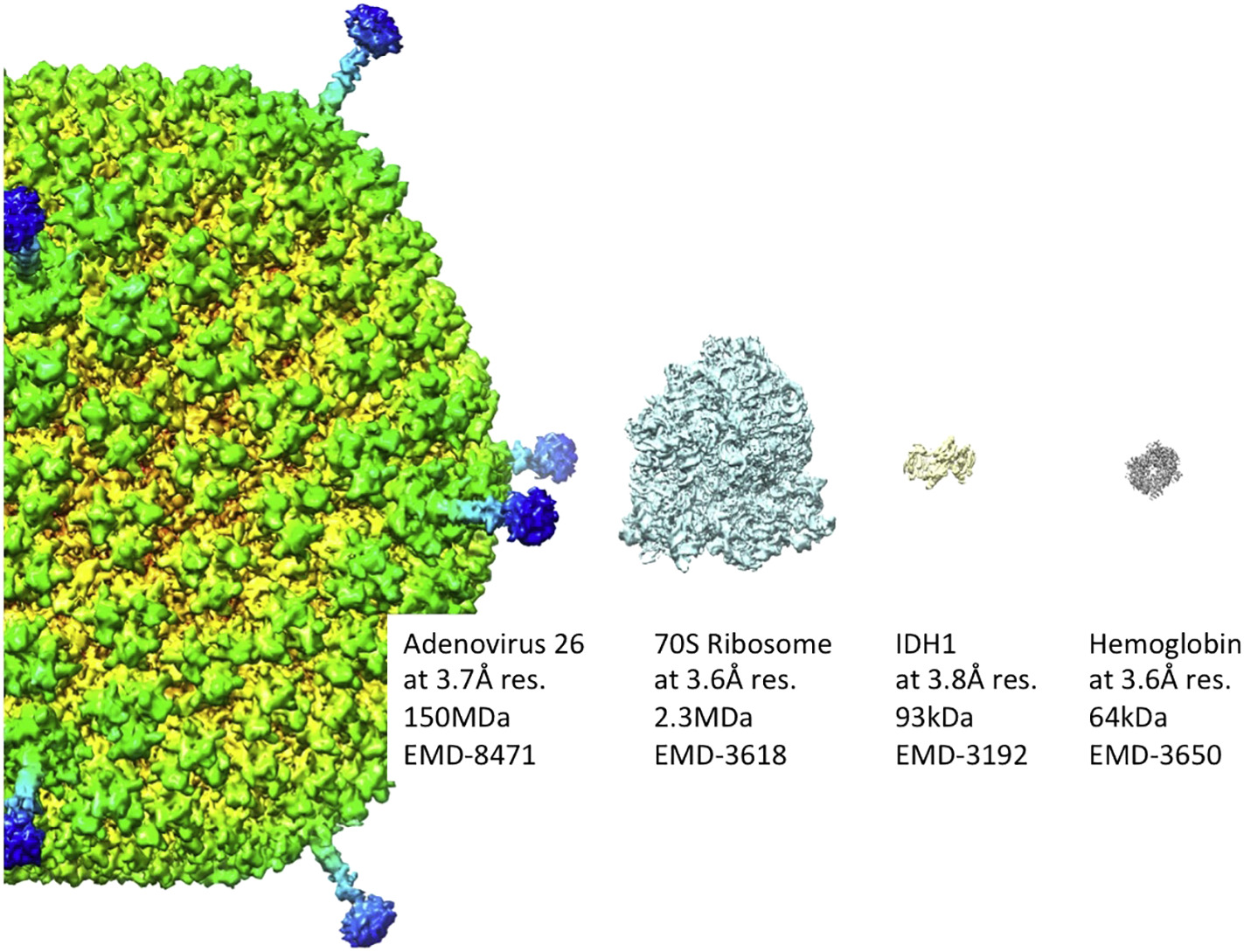
Figure 2: Near-atomic resolution representative images of biomolecules by cryoEM are shown. It shows that cryo-EM can cover a wide molecular weight range of specimens including protein complexes in the tens of kilo Daltons and large virus particles with hundreds of mega Daltons. Molecular weight and EM data base number are indicated for each particle (human adenovirus 26, 70S ribosome, isocitrate dehydrogenase 1 (IDH1), and human hemoglobin) [Source: Biochimica et Biophysica Acta (2018)].
Future Prospect in Drug Discovery
Major technological advances are anticipated in the near future development of direct electron detectors and more effective computational image analysis techniques. These are becoming instrumental for cryo-EM especially for application in solving high-resolution structures of pharmaceutically relevant large macromolecular assemblies. These protein structures are important for the pharmaceutical industry. Further, cryo-EM is being increasingly employed to investigate how ligand-binding sites, membrane proteins, both big and small, pseudo symmetry and complexes [7, 8]. All these developments show the promise of single-particle cryo-EM that is expected to become an important tool for drug discovery in the near future. Such advances are believed to enable structural determination for targets that are not accessible to X-ray crystallographic analysis [7].
The ongoing studies involve massive and continuing technology upgradations in hardware and software in cryo-EM. This is expected to expand the role of cryo-EM in providing an additional route to the structural information required to guide structure-based drug design, in addition to X-ray crystallography, NMR and homology modelling (Figure 3) [8].
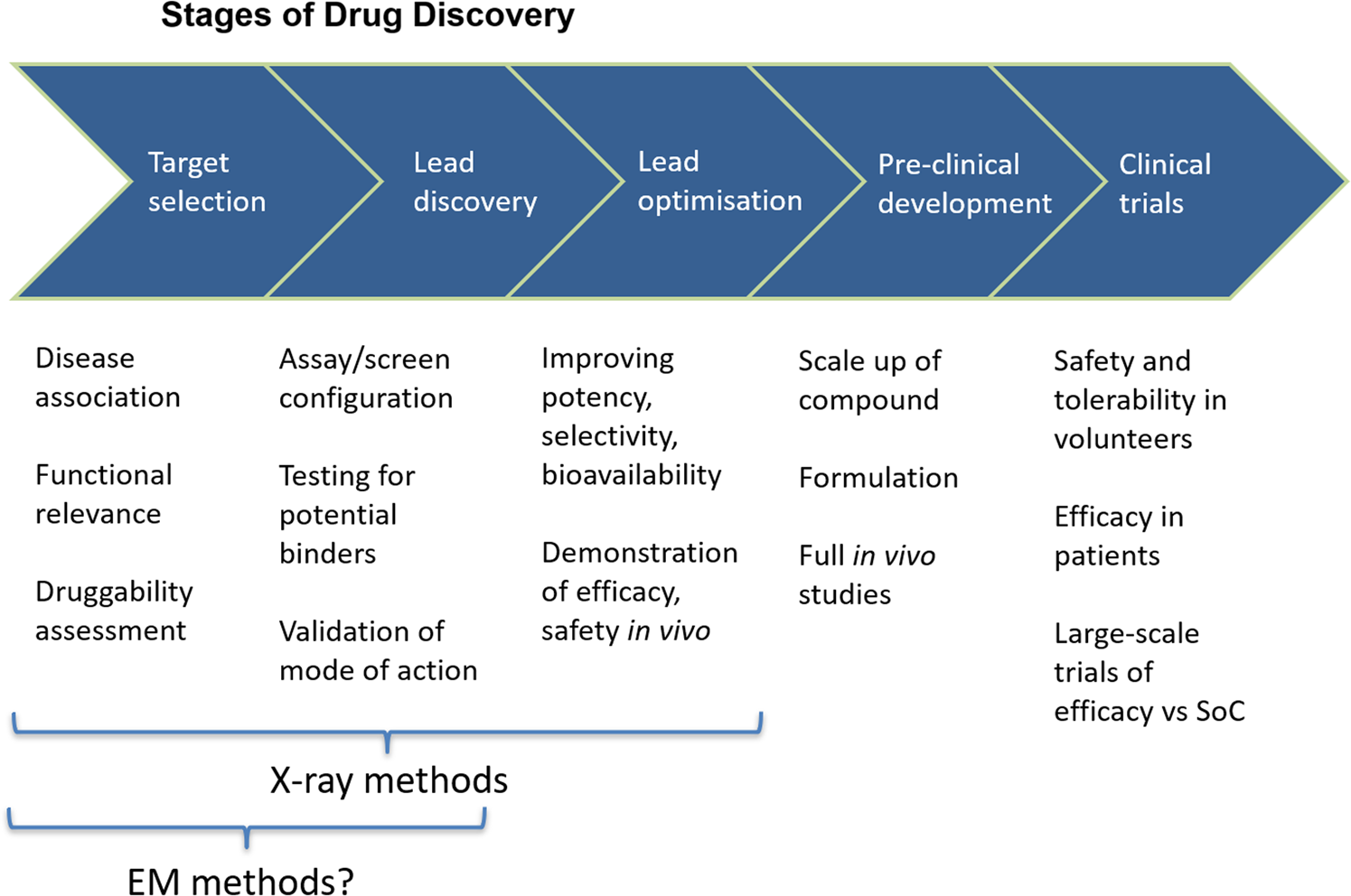
Figure 3: A conceptual pathway for cryogenic electron microscopy for drug discovery stages is shown. This pathway is compared with the X-ray crystallography that is heavily used in the lead discovery and lead optimization stages of a project [Source: Biochem Soc Trans (2019)].
In some of the ongoing studies, researchers have employed protein structure in all parts of the drug discovery process. This include the initial selection of protein to be targeted and the identification and optimization of chemical matter against the target. Additionally, a multi-threaded process is also involved by which the final properties of the compound are developed, which leads to the final clinical candidate (Figure 3) [8]. To achieve the goals, a chemical hit is usually considered the first visualization of a small molecule that is bound to the target protein. To this end, the technique based on crystallography has been used to examine the difference in electron density maps in such chemical hits. It is to be noted that the advances made in high-resolution EM maps offer the possibility for small compounds to be visualized directly, which is considered a significant step forward in the field of drug discovery by direct visualization of target molecules. Especially, it has been suggested that the confirmation of binding can be done by collecting data on close analogues [8].
Conclusion
We have presented a brief overview of some of the notable studies that have clearly shown the huge promise of cryogenic electron microscopy ‘cryo-EM’ as an emerging technology in the direct visualization of biomacromolecular assemblies. This could be immensely beneficial in the drug discovery industry. Especially, high vacuum technology has been shown instrumental that can be leveraged to make substantial advances in detector technology, microscopes and data processing in the operation of cryo-EM for a seamless transfer of frozen-hydrated biosamples. We anticipate that future studies would involve upgradation in the technology of cryo-EM that include superior direct electron detectors with back-thinning, electron counting to enhance detective quantum efficiency and a faster read-out rate can increase throughput and data collection times. All these advances are anticipated to lead to a new era of faster and efficient drug discovery pathway for the pharmaceutical industry.
References
[1] Tacke, S.; Krzyzanek, V.; Nüsse, H.; AlbertWepf, R.; Klingauf, J.; Reichelt, R. A Versatile High-Vacuum Cryo-transfer System for Cryo-microscopy and Analytics. Biophysical Journal, 2016, 110 (4): 758-765. DOI: https://doi.org/10.1016/j.bpj.2016.01.024.
[2] Dukes, M.; J.; Thomas, R.; Damiano, J.; Klein, K.; L.; Balasubramaniam, S.; Kayandan, S.; Riffle, J.; S.; Davis, R.; M.; McDonald, S.; M.; Kelly, DF. Improved microchip design and application for in situ transmission electron microscopy of macromolecules. Microsc Microanal., 2014, 20(2):338-45. DOI: https://doi.org/10.1017/S1431927613013858.
[3] Kaufmann, R.; Schellenberger, P.; Seiradake, E.; Dobbie, I.; M.; Jones, E.; Y.; Davis, I.; Hagen, C.; Grünewald, K. Super-resolution microscopy using standard fluorescent proteins in intact cells under cryo-conditions. Nano Lett., 2014, 9;14(7):4171-5. DOI: https://doi.org/10.1021/nl501870p.
[4] Murata. K.; Wolf, M. Cryo-electron microscopy for structural analysis of dynamic biological macromolecules. Biochim Biophys Acta Gen Subj., 2018, 1862(2):324-334. DOI: https://doi.org/10.1016/j.bbagen.2017.07.020.
[5] Herzik, M.; A.; Wu, M.; & Lander, G.; C. High-resolution structure determination of sub-100 kDa complexes using conventional cryo-EM. Nat Commun, 2019, 10:1032. DOI: https://doi.org/10.1038/s41467-019-08991-8.
[6] Khoshouei, M.; Radjainia, M.; Baumeister, W. et al. Cryo-EM structure of haemoglobin at 3.2 Å determined with the Volta phase plate. Nat Commun, 2017, 8:16099. DOI: https://doi.org/10.1038/ncomms16099.
[7] Renaud, J.; P.; Chari, A.; Ciferri, C. et al. Cryo-EM in drug discovery: achievements, limitations and prospects. Nat Rev Drug Discov, 2018, 17:471–492. DOI: https://doi.org/10.1038/nrd.2018.77.
[8] Ceska, T.; Chung, C.; W.; Cooke, R.; Phillips, C.; Williams, P.; A. Cryo-EM in drug discovery. Biochem Soc Trans., 2019, 47(1):281-293. DOI: https://doi.org/10.1042/BST20180267. Erratum in: Biochem Soc Trans. 2019 Feb 28;47(1):487.
Rights and Permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third[1]credit line to the permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Creative Commons This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. You are not required to obtain permission to reuse this article. To request permission for a type of use not listed, please contact Biotechnology Kiosk.