Surface Modification of Advanced Biomaterials for Applications in the Pharmaceutical and Medical Fields.
Ishita Agrawal1, Piyush Dua2*
1Department of Molecular and Human Genetics, Institute of Science, BHU, Varanasi, India.
2Department of Engineering, University of Technology and Applied Sciences, Suhar, Sultanate of Oman.
Abstract
Lately, there has been a great deal of emphasis on developing novel biomaterials for next generation biomedical technologies. Especially, research efforts have focused on biomaterials that meet the demand for precisely engineered three-dimensional structures. These research efforts seek to design advanced biomaterials that mimic the natural environments of tissues more closely, and thus enhance the functional performance of these materials. To this end, surface modification/functionalization of biomaterials is considered pivotal to achieve the goals. Recent progress in biomaterials fabrication techniques has shown huge promise for surface engineering of biomaterials leading to realization of devices that have complex surface geometries for various biomedical applications in the pharmaceutical and medical fields. These include next generation drug delivery, diagnosis and biosensors, to name a few. In this review, we have highlighted important surface modification processes that have been employed for surface engineering of biomaterials. Further, an overview of the cellular response of surface modified biomaterial is presented. We have also discussed precise engineering of three-dimensional surface modification of biomaterials by initiated chemical vapor deposition (i-CVD) method. Notable biomedical applications have been described. Finally, we have presented a brief future perspective.
Keywords: Biomaterials, i-CVD, surface modification, drug delivery, pathogen
*Corresponding author: Dr. Piyush Dua
E-mail address: piyushd.soh@cas.edu.om
DOI: https://doi.org/10.37756/bk.22.4.3.1
Article type: Review
Received: January 10, 2022
Revised: February 15, 2022
Accepted: February 17, 2022
Please cite this article as: Agrawal I and Dua P, Surface Modification of Advanced Biomaterials for Applications in the Pharmaceutical and Medical Fields, Biotechnol. kiosk, Vol 4, Issue 3, PP: 1-16 (2022); DOI: https://doi.org/10.37756/bk.22.4.3.1
Introduction
The ability to control the interactions between biomaterials and living tissues is considered critical to optimize their therapeutic effects and disease diagnostics for clinical applications. However, it is quite difficult to gain such ability because most biomaterials including metals, polymers, hydrogels, carbons, and composites do not exhibit specific surface and bulk properties and desirable functions that are suitable for applications. It is essential that biomaterials have perfect properties for their effective interactions with surrounding tissues. To overcome the challenges, surface modification/engineering of biomaterials has been shown to play an important role in tailoring the surface of biomaterials. This surface engineering allows better adaptation to the physiological surroundings and deliver the required clinical performance [1-3].
An important aspect in the rational design of biomaterials is to ensure surface modification of the biomedical devices without compromising their bulk characteristics for better control of the chemical and physical properties of the bioactive surfaces. Bulk properties are initially considered for a biomaterial’s suitability for an application. However, more important considerations are given to the physical aspects of the material surface as well as the chemistry that are critical to the function of many biomedical devices. Thus, physical and chemical surface modifications are of immense interest that seek to create a specific physical and chemical environment that offers a favorable cellular response in hard or soft tissue. For example, the physical environment including macro, micro, and even nanoscale features is considered in cases where tissue integration is desired. Creating a specific physical or chemical environment allows for cells to adhere, proliferate, and migrate [4].
Surface characteristics such as topographic and geometric features are especially targeted to gain the ability to regulate the cellular response. This allows to create specially designed bioscaffolds with specific surface functionalities via surface modification. This can subsequently offer several advantages compared to flat surface including cell adhesion and cell fate decision [5-14]. Pure chemical treatment of the material surface can result in oxideing/nitriding/carbiding a surface. It also includes surface functionalization as well as ion infusion. This can be achieved by single layer coatings, or multiple layers of coatings comprising different compositions [15-17].
Recent advances in surface modification techniques have allowed researchers to modify biomaterials to achieve required chemical and physical properties. These include biocompatibilities, surface functionalities, and mechanical strength that are sought in the field of tissue engineering, regenerative medicine, and biomedical devices. To this end, a range of surface engineering strategies are devised in order to achieve desired biocompatibility and other functionality such as antimicrobial performance in-situ. Several techniques have been employed that include plasma and chemical vapor deposition, atomic layer deposition, and electro-chemical deposition, to name a few [4, 14, 15, 18-24].
In this review, we have described surface modified biomaterials and the associated cellular response and strategies for surface modification/engineering. While several surface engineering strategies have been discussed in the literature, our emphasis is on the solvent-free processes especially the emerging i-CVD method for atomic scale engineering and its applications.
Surface Modified Biomaterial and the Associated Cellular Responses
Studies have shown that cellular responses such as cell adhesion, proliferation, differentiation and migration are dependent on surface microstructural topography along with the surface chemistry including surface interaction at the biomaterial–cell interface and surface energy/wettability [25-28]. Especially, changes in the biointerface have been shown to enable triggering specific cell signaling that can result in different cellular responses. Therefore, a major goal of the surface modification of biomaterials involves pathways that enable interaction with the surrounding tissues and biological fluids and elicit desired cellular responses (Figure 1) [20].
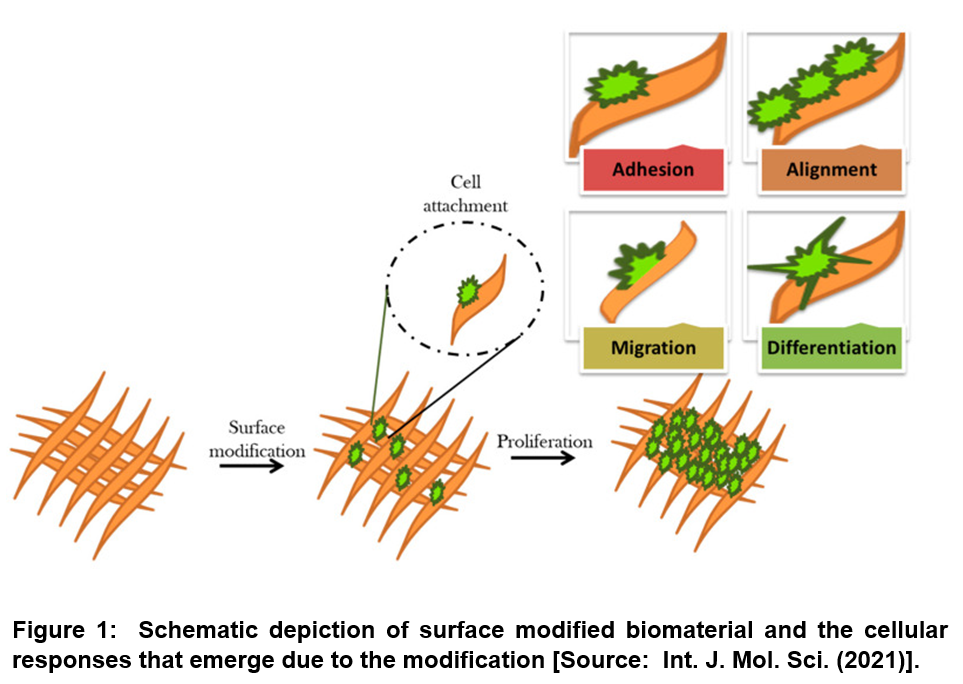
Researchers have shown that performance of biomaterials in living tissues can be altered depending on the types of biomolecule and bioactive agents along with properties of biomaterial surfaces that are applied for surface functionalization. Previous studies on surface modification effects have correlated the cellular behavior with the changes in surface chemistry, surface charge, hydrophilicity, surface topography, and softness and also stiffness of bio-materials. With respect to the connection of surface chemistry with wettability and surface charge, it has been shown that modified surface chemistry affects cell adhesion, cell shape, cell proliferation, and differentiation. In addition, surface chemistry has been shown to strongly affect materials’ biocompatibility as well as immunogenicity [20].
Studies have highlighted responsiveness of cells to the topographical structure of the underlying biomaterial surface. Accordingly, cells have been shown to modulate their alignment and orientation along the surface. The main components of surface topography include surface roughness and surface patterns that determine. The emphasis has been on unique properties of surface topography patterns such as high stability, cost-effective manufacture, and easy controllability for controlling cell function and tissue regeneration. With respect to the effects of surface charges, researchers have shown different mechanisms for solid surfaces to make them neutrally, positively, and negatively charged for different functions. For examples, more cells can be attached to the positively charged surface compared to the negatively and neutrally charged surfaces. Furthermore, it has been shown that the effects of surface charge on cellular responses depend on the composition of biomaterials, cell type as well as tissue microenvironment. Regarding surface wettability and its effects, it is known that wettability features such as hydrophilicity and hydrophobicity correspond to the adhesive force between the liquid and solid material surface that results in the spreading of the liquid across a solid surface. Several studies have documented that while cells are typically attached and proliferated on a hydrophilic surface, proteins tend to bind onto hydrophobic surfaces. Last but not the least is the consideration on surface energy and cellular responses. Surface energy is recognized as one of the decisive factors for surface wettability of biomaterials. For example, Surfaces with low surface free energy have been shown to be less adhesive than those with high surface free energy. Especially for tissue engineering applications, it has been sown that biomaterials with total surface energies of about 100–129 erg cm−2 are more suitable for tissue regeneration. Further, the non-optimal range of total surface energies is thought to be within about 16–20 erg cm−2 to support cell adhesion, proliferation, and differentiation. The stiffnesses of underlying substrate and local extracellular matrix have also been considered to be guiding factors for cell morphology and fate decision [20, 29, 30].
Conventional and Emerging Surface Engineering Strategies for Biomaterials
From a purely materials perspective, surface engineering is often considered to overcome the challenges of loss of quality of a material due to fatigue/fracture, wear and destruction as a result of mechanical sliding interaction and corrosion or decorative defects. Previous studies showed surface engineering strategies helpful in inducing surface tolerant properties to combat detrimental environmental conditions or external forces [31-33]. With respect to biomaterials, surface engineering has been shown to impart cell adhesion, passage, growth, differentiation and also functionality. Surface engineering can also influence roughness that can be leveraged to control the effectiveness of coating [20, 34, 35].
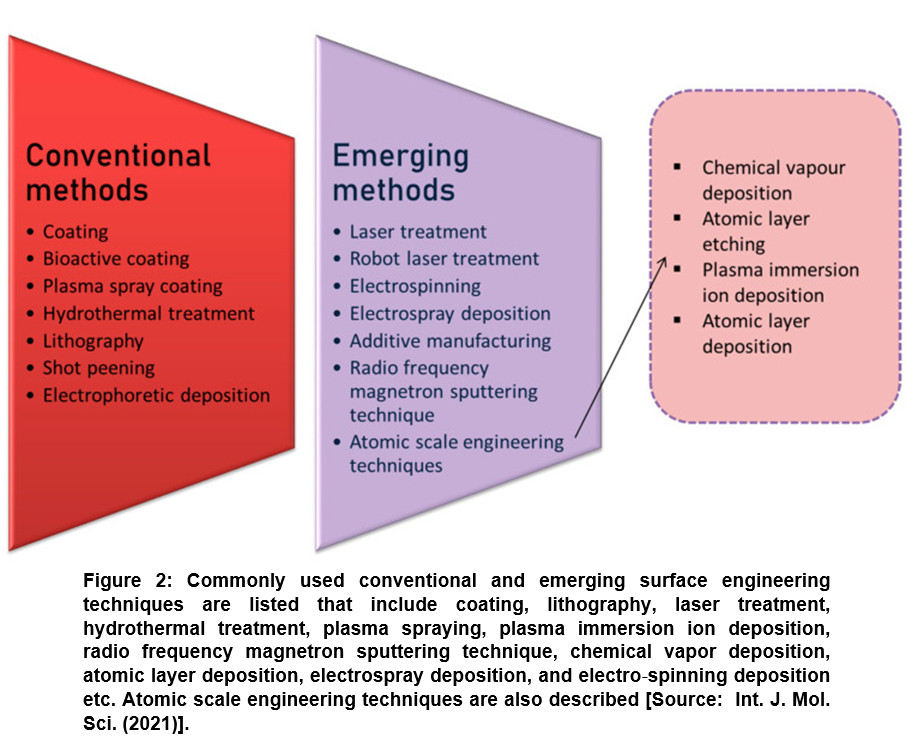
Several nanofabrication and microfabrication techniques for applying surface topography have been considered that include electron beam lithography or photolithography; replica casting or molding; self-assembling systems; particle synthesis; microcontact printing; sandblasting; electrospinning; and chemical etching etc. For simplicity, surface engineering methods have been divided in two categories. These are conventional surface engineering methods that deal with coating, bioactive coating, plasma spray coating, hydrothermal, lithography, shot peening, and electrophoretic deposition and emerging surface engineering methods such as laser treatment, robot laser treatment, electrospinning, electrospray, additive manufacturing, and radio frequency magnetron sputtering technique) (Figure 2) [20].
Lately, researchers have paid much more attention to emerging surface modification methods that are considered more advanced and innovative techniques. Emerging methods are considered advantageous because they can be employed for obtaining improved biocompatibility that is otherwise very difficult to achieve by conventional modifying techniques. Most recent advances point towards machine learning and atomic scale engineering techniques [36]. Machine learning methods are rather in a very nascent stage, where a computer learns the information. This subsequently provides data and the system functions according to the data. On the other hand, atomic scale surface engineering is a series of methods that are considered most promising emerging techniques to alter the surface topography at the atomic and molecular scale (<100 nm) (Figure 2) [20]. These techniques are currently driving the biomaterial research that deals with fabricating a material with improved understanding of surface interactions by modifying internal components. Further, from pure applications point of view, atomic scale engineering can be leveraged for designing a commercial product with excellent antimicrobial and other biomedical functional properties for applications in advanced pharmaceutics and medicine [37-42].
In the following sections, we will describe the main process of initiated chemical vapor deposition ‘i-CVD’ for the surface modification of biomaterials along with notable applications of i-CVD.
Initiated Chemical Vapor Deposition: Atomic Scale Engineering of Three-Dimensional Surface Modification of Biomaterials
Atomic-scale engineering has recently emerged very promising for three-dimensional (3D) surface modification of biomaterials. Techniques such as chemical vapor deposition, atomic layer etching, plasma immersion ion deposition, and atomic layer deposition correspond to the subsection of emerging technology that has been shown superior over conventional methods for improved control and flexibility at finer length scales. Recent advances in technologies have shown promise to meet the demand for better control of biomaterial surfaces. These advances are aimed at the atomic scale and molecular scale engineering while incorporating functional bio-active agents for enhanced in-situ performance of new biomedical devices such as implants. Several studies have used functional agents that include synthetic materials such as monolithic ZnO, quaternary ammonium salts, silver nanoclusters, titanium dioxide, and graphene and also natural materials, for example, chitosan, totarol, botanical extracts, and nisin for atomic scale engineering [43-47].
Recently, iCVD has emerged as a top-of-the-line have technique for atomic scale engineering and as a damage-free method for sensitive biomaterials to modify the surface of biomaterials and various biomedical devices in a controlled fashion. The technique i-CVD is considered highly beneficial for the conformal deposition of various functional biopolymer films. This deposition can be done onto many kinds of bio-surfaces without restrictions on the substrate material or geometry, which is otherwise not possible to achieve by conventional solution based surface functionalization methods. It has been shown that with proper structural design, it is possible to achieve required functionality to the biomaterial surfaces by the functional polymer thin film via i-CVD. Such functionality can be achieved while maintaining the fine structure thereon [48].
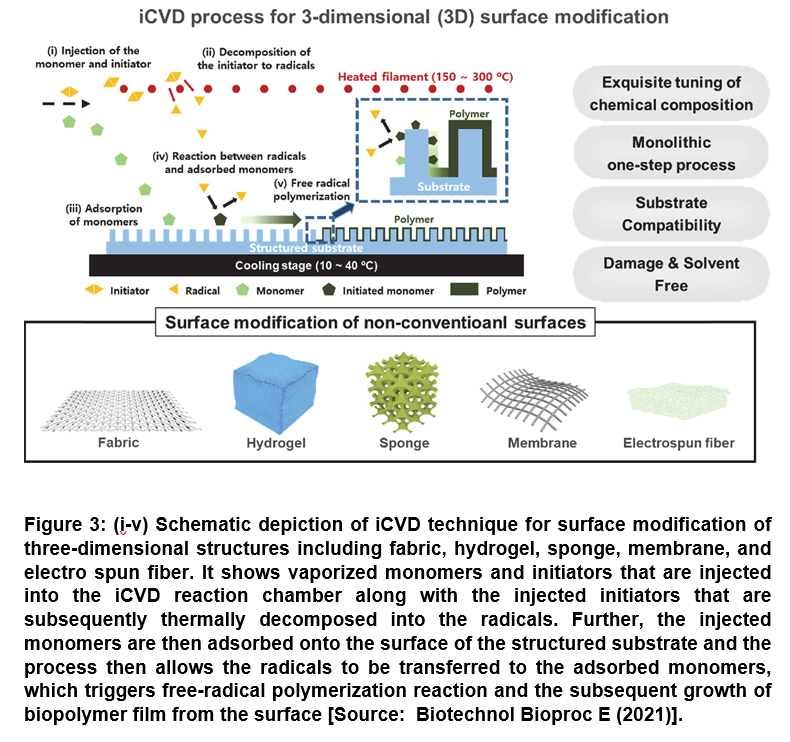
The technique iCVD offers a number of advantages that include the mild deposition condition and also the deposition process that can be proceeded in solvent-free and near-room temperature condition. In a typical iCVD process, the initiator and monomers are first vaporized and introduced simultaneously into the vapor phase reactor that is maintained under vacuum. Subsequently, the injected monomers are adsorbed onto the surface of substrates, while the initiators decomposed thermally via hot filament to form radicals. This results in triggering free-radical polymerization reaction at the substrate surface that is maintained near at room temperature (less than 50oC). This finally leads to the biopolymer or biomaterial film growth from the surface (Figure 3) [48].
The surface modification techniques based on iCVD process have been shown to be capable of engineering the surface of the non-conventional substrates as well, which allows for the rational design of biomaterial platforms. This subsequently enables to control the adsorption/immobilization of bioactive molecules to broaden the utility of biomaterials for a wide range of advanced technical approaches for biomedical applications. Therefore, the developments in atomic scale surface engineering using iCVD have proven to be very useful in the field of biomedical applications by realizing conformal coating of biological and medical devices have are comprised of a range of miniature structures [48].
i-CVD Technique for Controlled Drug Delivery
There have been significant research efforts for safe drug delivery through mucosae. Such drug delivery offers advantageous characteristics including accurate dose control and the avoidance of premature metabolism of vulnerable drugs by oral administration. However, there is a challenge involving body fluid in mucosae that may dissolve the drug and releasing it to unwanted directions [49-51].
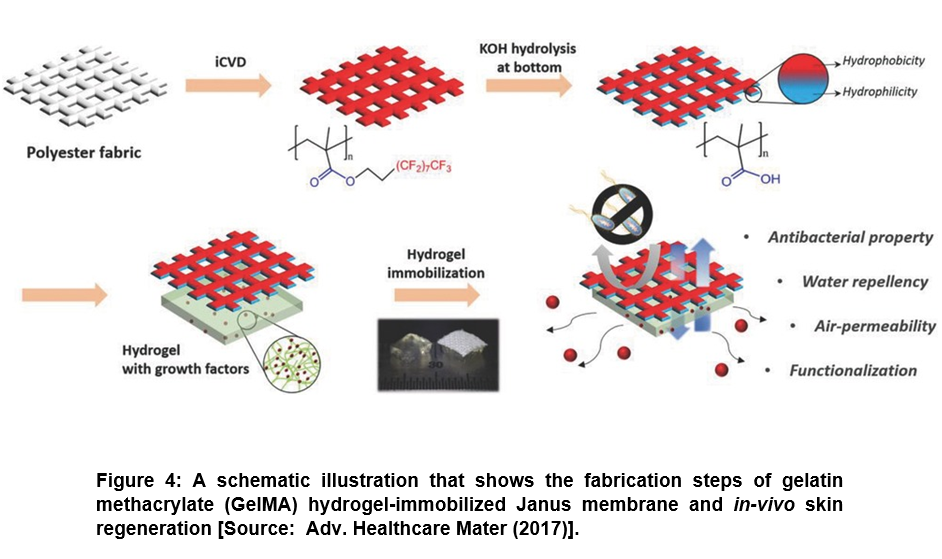
To address the challenge of unwanted dissolving of drug, researchers showed the promise of iCVD technique to control the drug delivery rate and direction to the target region by area selective incorporation of surface functionality. They used area-selective functionalization of membrane to provide Janus property that exhibited a great potential for drug delivery applications. To this end, a Janus patch was developed for mono-directional drug delivery. In this process, a polyester fabric was coated with poly(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecyl methacrylate) (pHFDMA) film by iCVD process (Figure 4) [48, 52]. Subsequently, base-catalyzed hydrolysis was applied to one side of the substrate that rendered hydrophilic surface containing carboxylic acid residues. Further, while the hydrophilic surface allowed the coating of hydrogel incorporated with resveratrol that enhanced the adhesion of patch to mucosa, the hydrophobic surface prevented wetting by body fluids. It was shown in this study that the developed Janus patch enabled controlling the exact dose with intended directional drug release without allowing the water penetration. Further, researchers showed the applicability of this approach to various kinds of porous materials such as Nylon mesh or paper and not restricted to polyester fabric only [48, 52].
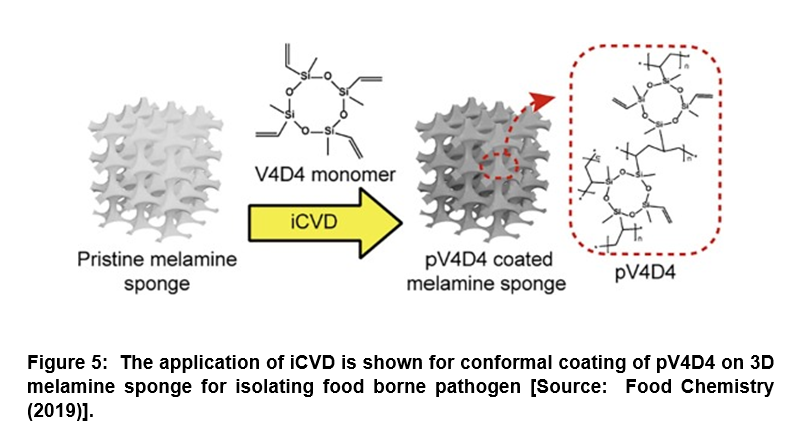
Surface-Modified Porous Sponge for Isolating Foodborne Pathogen
It is widely recognized that rapid and efficient detection of pathogenic bacteria from food is critically important to prevent epidemic food poisoning. However, the challenges lie in the isolation of pathogenic bacteria from spoiled food, which is further hampered by the lack of proper cell cultivation and/or isolation methods [53, 54]. Conventional methods suffer from complex, time-consuming culturing steps that result in low scalability, and high operation cost. To overcome these challenges, researchers demonstrated an alternative approach for the isolation of pathogenic bacteria directly from food using a surface-modified, highly porous sponge via iCVD process (Figure 5) [55]. In this study, a hydrophobic polymer, poly(2,4,6,8-tetravinyl-2,4,6,8-tetramethyl cyclotetra-siloxane) (pV4D4) was deposited conformally by iCVD on amphiphilic three-dimensional (3D) melamine sponge. This was done to incorporate hydrophobicity as well as oleophilicity to the porous sponge surface for absorbing oil component selectively from food extracts. Researchers demonstrated that the surface-modified sponge was capable of the isolation of Escherichia coli O157:H7 (E. coli O157:H7) from heterogeneous mixture with oil/water/food particles with high efficiency compared to artificial model system. The surface-modified sponge developed could pave the way for the development of a novel biotechnology platform for oil/water separation and isolation of foodborne pathogens directly from heterogeneous mixture that could enhance the efficiency of molecular diagnostics [55].
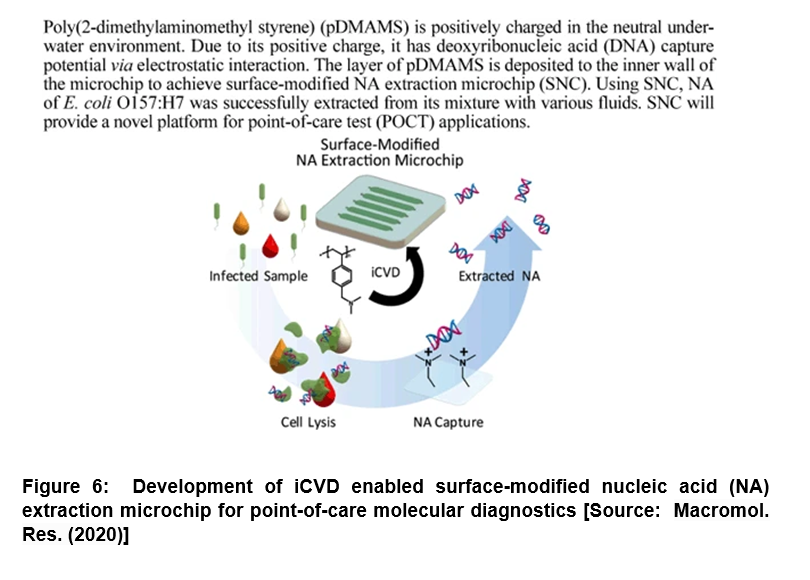
Modification of Microchip for DNA Extraction: Enhanced Capturing Efficiency for Point-of-Care Molecular Diagnostics
It has been shown that nucleic acid (NA) extraction and purification corresponds to one of the most important steps for NA-based molecular diagnosis. However, the conventional methods have severe limitations due to many issues that include long processing time, complicated steps, requirement of trained personnel and potential inhibition caused by chaotropic agents and/ or residual reagents [56-59]. To address these challenges, researchers demonstrated a surface-modified NA extraction microchip (SNC) by introducing poly(2-dimethylaminomethyl styrene) (pDMAMS) film engaged directly on the microchip surface via iCVD process (Figure 6) [60]. In this study, the positively charged SNC inner surface was shown to directly capture the negatively charged NA efficiently. Its performance was confirmed by fluorescence microscopy and X-ray photoelectron spectroscopy. Researchers showed that the developed SNC exhibited the deoxyribonucleic acid (DNA) capture efficiency higher than 92% regardless of initial DNA concentration in range of 20 ng/µL to 0.01 ng/µL. Further, with this versatile DNA-capturing surface, researchers successfully extracted the genomic DNAs of Escherichia Coli O157:H7 (E. coli O157:H7) directly from cell lysate in the SNC with higher than 90% of efficiency within 30 min. The extraction time was shown to reduce to at least of 10 min by simply maintaining extraction efficiency higher than 50%. Further, the genomic DNAs were directly extracted using the SNC that involved no loss from various real samples including juice, milk and blood serum. Thus, the proposed SNC could be very useful in future studies that could pave the way to new avenues to perform an one-step NA extraction for molecular diagnosis with the potential to be integrated into a micro-total analysis in the fields of point-of-care diagnosis [60].
Conclusion & Future Perspective
We have described surface modification/engineering methods that are increasingly being employed in three-dimensional surface modification of clinically grade biomaterials. Surface engineering of biomaterials is considered pivotal in preserving the functional attributes of biomaterial. Studies have shown that it can be leveraged to enhance its effectiveness and sustainability by adding a functional layer to the surface. In addition, changing the surface texture is another viable route for effective surface modification of biomaterials. It has been shown that such surface engineering could help prevent corrosion and erosion and also promotes osseointegration while enhancing biocompatibility especially in tissue engineering applications. In addition, surface modification has been shown to induce self-healing property, resists friction and wear, and improve cell adhesion while reducing thrombogenicity that results in desired transport characteristics, and minimizes the risk of microbial infections.
Recent research advances in initiated chemical vapor deposition (i-CVD) for biofunctionalization of biopolymer thin films have shown tremendous promise in pharmaceutical and medical fields. Several studies have shown notable advances in the field of i-CVD biopolymer films on different substrate materials that include immobilization of bioactive molecules, site-specific conjugation, and facile control of physio-chemical property of implants and biomedical devices. Studies have also demonstrated the deposition of biopolymeric thin films in vapor phase monomers that can substantially facilitate adjusting the polymer compositions. This is believed to be a hallmark of i-CVD technique that allows precise topological and chemical control of medical implants with complex three-dimensional shapes. However, the development of i-CVD functional polymer coatings for various medical devices is still considered in its early stage. In future studies, i-CVD technique is expected to make significant contributions in the design and production of superior biological and medical devices. This could be achieved by providing substrate-independent polymer coatings with nondestructive way. We anticipate that future studies will also include further development and optimization of i-CVD technique that will allow mass production and quality control of polymer-coated medical device for seamless transition of laboratory investigation to clinical practice. To this end, in term of substrate-independent polymer coatings with nondestructive way, iCVD technique is expected to make more significant contributions in the design and production of advanced biological and medical devices.
Another area of future interest is in-depth biological assessment of polymer-coated surface that is required for direct surface contact of cell/tissue. This is to gain new insights into the biomolecular mechanism underlying the series of cellular response on the functional polymer surface. This will lead to a deeper investigation on the interactions of i-CVD polymer-functionalized surface and cell/tissue surface that will provide great advantages for the rational design of the surface chemistry and develop elaborate medical devices responding to unmet medical needs. It is believed that compared to conventional solution-based processes, the advantageous i-CVD process will help overcome the technical challenges that are often faced in nonclinical and clinical pre-market assessments in both device manufacture and functional performance.
With respect to next-generation bioscaffold designs, the application of biomaterials may focus on addressing concerns with respect to their biodegradability and cell toxicity. This could be a major goal especially in view of the still unknown prolonged in-vivo cytotoxicity, effectiveness, and fate of surface engineered scaffolds. Thus, it may shed light on understanding the surface properties of scaffolds along with their effects on cellular behavior. This could help overcome the technical barriers toward their design of better scaffolds. In addition, future studies could involve surface modification of scaffolds by bioactive molecules. This could be achieved by creating substrates with desired properties that could pave the way to future breakthroughs involving instructing cellular behavior in terms of attachment, proliferation, and differentiation. In summary, it would be prudent to focus on research that enables greater insights of long-term in-vivo fate of surface engineered biomaterials that could be leveraged to further expand this exciting field for transition from laboratory to safe clinical applications.
References
[1] Guosong Wu, Penghui L, Hongqing F, Xuming Z and Paul KC, Engineering and functionalization of biomaterials via surface modification. J. Mater. Chem. B 2015, 3:2024-2042. DOI: https://doi.org/10.1039/C4TB01934B
[2] Mahajan A & Sidhu SS, Surface modification of metallic biomaterials for enhanced functionality: a review. Materials Technology 2018, 33:2, 93-105. DOI: https://doi.org/10.1080/10667857.2017.1377971
[3] Shuxiang C, Chuanxiang Wu, Wenguang Y, Wenfeng L, Haibo Yu, and Lianqing L, Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnology Reviews 2020, 9(1):971-989. DOI: https://doi.org/10.1515/ntrev-2020-0076.
[4] Bose S, Robertson SF, Bandyopadhyay A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater 2018, 15;66:6-22. DOI: https://doi.org/10.1016/j.actbio.2017.11.003.
[5] Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318(5849):426-30. DOI: https://doi.org/10.1126/science.1147241.
[6] Park HK, Lee D, Lee H and Hong S, A nature-inspired protective coating on soft/wet biomaterials for SEM by aerobic oxidation of polyphenols, Mater. Horiz 2020, 7:1387-1396. DOI: https://doi.org/10.1039/C9MH01448A.
[7] Arkhangelskiy A, Maniglio D, Bucciarelli A, Yadavalli VK, and Quaranta A, Plasma-Assisted Deposition of Silk Fibroin on Different Surfaces, Adv. Mater. Interfaces 2021, 2100324. DOI: https://doi.org/10.1002/admi.202100324.
[8] Maniglio D, Bonani W, Migliaresi C, Motta A. Silk fibroin porous scaffolds by N2O foaming. J Biomater Sci Polym Ed 2018, 29(5):491-506. DOI: https://doi.org/10.1080/09205063.2018.1423811.
[9] Wang C, Fang H, Qi X, Hang C, Sun Y, Peng Z, Wei W, Wang Y. Silk fibroin film-coated MgZnCa alloy with enhanced in vitro and in vivo performance prepared using surface activation. Acta Biomater 2019, 91:99-111. DOI: https://doi.org/10.1016/j.actbio.2019.04.048.
[10] Luo Y, Kang KB, Sartaj R, Sun MG, Zhou Q, Guaiquil VH, Rosenblatt MI. Silk films with nanotopography and extracellular proteins enhance corneal epithelial wound healing. Sci Rep 2021,11(1):8168. DOI: https://doi.org/10.1038/s41598-021-87658-1.
[11] Sensharma P, Madhumathi G, Jayant RD, Jaiswal AK. Biomaterials and cells for neural tissue engineering: Current choices. Mater Sci Eng C Mater Biol Appl 2017, 77:1302-1315. DOI: https//doi.org/10.1016/j.msec.2017.03.264.
[12] Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22(3):340-354. DOI: https://doi.org/10.1016/j.stem.2018.02.009. Erratum in: Cell Stem Cell 2018, 22(4):608.
[13] Raval N, Kalyane D, Maheshwari R, Tekade RK, Chapter 17 – Surface Modifications of Biomaterials and Their Implication on Biocompatibility, Editor(s): Rakesh K. Tekade, In Advances in Pharmaceutical Product Development and Research. Biomaterials and Bionanotechnology, Academic Press 2019, 639-674, ISBN 9780128144275. DOI: https://doi.org/10.1016/B978-0-12-814427-5.00017-2.
[14] Roy M, Bandyopadhyay A, Bose S, Induction Plasma Sprayed Nano Hydroxyapatite Coatings on Titanium for Orthopaedic and Dental Implants. Surface & Coatings Technology 2011, 205(8-9):2785–2792. DOI: https://doi.org/10.1016/j.surfcoat.2010.10.042.
[15] Roy M, Fielding GA, Beyenal H, Bandyopadhyay A, Bose S. Mechanical, in vitro antimicrobial, and biological properties of plasma-sprayed silver-doped hydroxyapatite coating. ACS Appl Mater Interfaces. 2012, 4(3):1341-9. DOI: https://doi.org/10.1021/am201610q.
[16] Vahabzadeh S, Roy M, Bandyopadhyay A, Bose S, Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater 2015, 17:47-55. DOI: https://doi.org/10.1016/j.actbio.2015.01.022.
[17] Lim JY, Donahue HJ, Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng 2007, 13(8):1879-91. DOI: https://doi.org/10.1089/ten.2006.0154.
[18] O’Shaughnessy WS, Gao M, Gleason KK, Initiated Chemical Vapor Deposition of Trivinyltrimethylcyclotrisiloxane for Biomaterial Coatings. Langmuir 2006, 22(16):7021–7026. DOI: https://doi.org/10.1021/la0607858.
[19] Yu, S. J., K. Pak, M. J. Kwak, M. Joo, B. J. Kim, M. S. Oh, J. Baek, H. Park, G. Choi, D. H. Kim, J. Choi, Y. Choi, J. Shin, H. Moon, E. Lee, and S. G. Im (2018) Initiated chemical vapor deposition: a versatile tool for various device applications. Adv. Eng. Mater. 20: 1700622.
[20] Sultana, A.; Zare, M.; Luo, H.; Ramakrishna, S. Surface Engineering Strategies to Enhance the In Situ Performance of Medical Devices Including Atomic Scale Engineering. Int. J. Mol. Sci. 2021, 22, 11788, DOI: https://doi.org/10.3390/ ijms222111788.
[21] Kim YS, Raston NH, Gu MB, Aptamer-based nanobiosensors. Biosens Bioelectron 2016, 76:2-19. DOI: https://doi.org/10.1016/j.bios.2015.06.040.
[22] Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT, Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 2015, 63:218-231. DOI: https://doi.org/10.1016/j.bios.2014.07.029.
[23] Amani H, Arzaghi H, Bayandori M, Dezfuli AS, Pazoki-Toroudi H, Shafiee A, Moradi L, Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6:1900572, DOI: https://doi.org/10.1002/admi.201900572.
[24] Chu PK, Chena JY, Wang LP, Huang N, Plasma-surface modification of biomaterials. Materials Science and Engineering: R: Reports 2002, 36:143–206, DOI: https://doi.org/10.1016/S0927-796X(02)00004-9.
[25] Tu CX, Gao CY, Recent Advances on Surface-modified Biomaterials Promoting Selective Adhesion and Directional Migration of Cells. Chin J Polym Sci 2021, 39:815–823. DOI: https://doi.org/10.1007/s10118-021-2564-5.
[26] Feller L, Jadwat Y, Khammissa RA, Meyerov R, Schechter I, Lemmer J, Cellular responses evoked by different surface characteristics of intraosseous titanium implants. Biomed Res Int 2015, 2025:171945. DOI: https://doi.org/10.1155/2015/171945.
[27] Boyan B. D., Sylvia V. L., Liu Y., et al., Surface roughness mediates its effects on osteoblasts via protein kinase A and phospholipase A2. Biomaterials 1999, 20(23-24):2305–2310. DOI: https://doi.org/10.1016/s0142-9612(99)00159-3.
[28] Mantz A, Pannier AK, Biomaterial substrate modifications that influence cell-material interactions to prime cellular responses to nonviral gene delivery. Exp Biol Med (Maywood) 2019, 244(2):100-113. DOI: https://doi.org/10.1177/1535370218821060.
[29] Alves NM, Pashkuleva I, Reis RL, Mano JF, Controlling cell behavior through the design of polymer surfaces. Small 2010, 6(20):2208-20. DOI: https://doi.org/10.1002/smll.201000233.
[30] Mariani E, Lisignoli G, Borzì RM, Pulsatelli L, Biomaterials: Foreign Bodies or Tuners for the Immune Response? International Journal of Molecular Sciences 2019, 20(3):636. DOI: https://doi.org/10.3390/ijms20030636
[31] Rashidi H., Yang J, Shakesheff K.M. Surface engineering of synthetic polymer materials for tissue engineering and regenera-tive medicine applications. Biomater. Sci 2014, 2:1318–1331. DOI: https://doi.org/10.1039/C3BM60330J.
[32] Nathanael AJ, Oh TH, Biopolymer Coatings for Biomedical Applications. Polymers (Basel) 2020, 12(12):3061. DOI: https://doi.org/10.3390/polym12123061.
[33] Char DS, Shah NH, Magnus D, Implementing Machine Learning in Health Care – Addressing Ethical Challenges. N Engl J Med 2018, 378(11):981-983. DOI: https://doi.org/10.1056/NEJMp1714229.
[34] Ebrahiminezhad A, Raee MJ, Manafi Z, Jahromi AS, Ghasemi Y, Ancient and Novel Forms of Silver in Medicine and Biomedicine. J. Adv. Med Sci. Appl. Technol 2016, 2:122–128. DOI: https://doi.org/10.18869/nrip.jamsat.2.1.122.
[35] Baranwal A, Srivastava A, Kumar P, Bajpai VK, Maurya PK, Chandra P, Prospects of Nanostructure Materials and Their Composites as Antimicrobial Agents. Front Microbiol 2018, 9:422. DOI: https//doi.org/10.3389/fmicb.2018.00422.
[36] Nene A, Galluzzi M, Hongrong L, Somani P, Ramakrishna S, Yu XF, Synthetic preparations and atomic scale engineering of silver nanoparticles for biomedical applications. Nanoscale 2021, 13(33):13923-13942. DOI: https//doi.org/10.1039/d1nr01851e.
[37] Narayan R, Goering P, Laser micro- and nanofabrication of biomaterials. MRS Bull 2011, 36:973–982. DOI: https://doi.org/10.1557/mrs.2011.302.
[38] Boda SK, Li X, Xie J, Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J Aerosol Sci 2018, 125:164-181. DOI: https://doi.org/10.1016/j.jaerosci.2018.04.002.
[39] Johnson RW, Hultqvist A, Bent SF, A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17:236–246. DOI: https://doi.org/10.1016/j.mattod.2014.04.026.
[40] Su C, Hu Y, Song Q, Ye Y, Gao L, Li P, Ye T, Initiated chemical vapor deposition of graded polymer coatings enabling anti-bacterial, antifouling, and biocompatible surfaces. ACS Appl. Mater. Interfaces 2020, 12:18978–18986. DOI: https://doi.org/10.1021/acsami.9b22611.
[41] Sun L, Yuan G, Gao L, Yang J, Chhowalla M, Gharahcheshmeh MH, Gleason KK, Choi YS, Hong BH, Liu Z, Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1:1–20. DOI: https://doi.org/10.1038/s43586-020-00005-y.
[42] Fang C, Cao Y, Wu D, Li A, Thermal atomic layer etching: Mechanism, materials and prospects. Prog. Nat. Sci. Mater. Int. 2018, 28:667–675. DOI: https://doi.org/10.1016/j.pnsc.2018.11.003.
[43] Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A, Engineering microscale topographies to control the cell-substrate interface. Biomaterials 2012, 33(21):5230-46. DOI: https://doi.org/10.1016/j.biomaterials.2012.03.079.
[44] Ayala R, Zhang C, Yang D, Hwang Y, Aung A, Shroff SS, Arce FT, Lal R, Arya G, Varghese S, Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32(15):3700-11. DOI: https://doi.org/10.1016/j.biomaterials.2011.02.004.
[45] Yim EK, Darling EM, Kulangara K, Guilak F, Leong KW, Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010, 31(6):1299-306. DOI: 10.1016/j.biomaterials.2009.10.037.
[46] Kim SH, Lee HR, Yu SJ, Han ME, Lee DY, Kim SY, Ahn HJ, Han MJ, Lee TI, Kim TS, Kwon SK, Im SG, Hwang NS, Hydrogel-laden paper scaffold system for origami-based tissue engineering. Proc Natl Acad Sci USA 2015, 112(50):15426-31. DOI: https://doi.org/10.1073/pnas.1504745112.
[47] Im SG, Bong KW, Lee CH, Doyle PS, Gleason KK, A conformal nano-adhesive via initiated chemical vapor deposition for microfluidic devices. Lab Chip 2009, 9(3):411-6. DOI: https://doi.org/10.1039/b812121d.
[48] Cho Y, Lee M, Park S et al., A Versatile Surface Modification Method via Vapor-phase Deposited Functional Polymer Films for Biomedical Device Applications. Biotechnol Bioproc E 2021, 26:165–178. DOI: https://doi.org/10.1007/s12257-020-0269-1.
[49] You JB, Choi AY, Baek J, Oh MS, Im SG, Lee KE, and Gwak HS, Application of monodirectional Janus patch to oromucosal delivery system. Adv. Healthcare Mater 2015, 4: 2229–2236, DOI: https://doi.org/10.1002/adhm.201500416.
[50] Sayin S, Tufani A, Emanet M, Genchi GG, Sen O, Shemshad S, Ozdemir E, Ciofani G, Ozaydin Ince G, Electrospun Nanofibers With pH-Responsive Coatings for Control of Release Kinetics. Front Bioeng Biotechnol 2019, 7:309. DOI: https://doi.org/10.3389/fbioe.2019.00309.
[51] Bedair TM, Yu SJ, Im SG, Park BJ, Joung YK, Han DK, Effects of interfacial layer wettability and thickness on the coating morphology and sirolimus release for drug-eluting stent. J Colloid Interface Sci 2015, 460:189-99. DOI: https://doi.org/10.1016/j.jcis.2015.08.051.
[52] An YH, Yu SJ, Kim IS, Kim SH, Moon JM, Kim SL, Choi YH, Choi JS, Im SG, Lee KE, Hwang NS, Hydrogel Functionalized Janus Membrane for Skin Regeneration. Adv Healthc Mater 2017, 6(5). DOI: https://doi.org/10.1002/adhm.201600795.
[53] Law JW, Ab Mutalib NS, Chan KG, Lee LH, Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 2015, 5:770. DOI: https://doi.org/10.3389/fmicb.2014.00770.
[54] Li X, Ximenes E, Amalaradjou MA, Vibbert HB, Foster K, Jones J, Liu X, Bhunia AK, Ladisch MR, Rapid sample processing for detection of food-borne pathogens via cross-flow microfiltration. Appl Environ Microbiol 2013, 79(22):7048-54. DOI: https://doi.org/10.1128/AEM.02587-13.
[55] Choi Y, Kim YT, You JB, Jo SH, Lee SJ, Im SG, Lee KG, An efficient isolation of foodborne pathogen using surface-modified porous sponge. Food Chem 2019, 270:445-451. DOI: https://doi.org/10.1016/j.foodchem.2018.07.125.
[56] Jones K, Patel N, Levy M et al., Global trends in emerging infectious diseases. Nature 2008, 451:990–993. DOI: https://doi.org/10.1038/nature06536.
[57] Gan W, Gu Y, Han J, Li CX, Sun J, Liu P, Chitosan-modified filter paper for nucleic acid extraction and “in situ PCR” on a thermoplastic microchip. Analytical chemistry 2017, 21;89(6):3568-75, DOI: https://doi.org/10.1021/acs.analchem.6b04882.
[58] Kim SH, Lee HR, Yu SJ, Han ME, Lee DY, Kim SY, Ahn HJ, Han MJ, Lee TI, Kim TS, Kwon SK, Hydrogel-laden paper scaffold system for origami-based tissue engineering. Proceedings of the National Academy of Sciences 2015,112(50):15426-31. DOI: https://doi.org/10.1073/pnas.1504745112.
[59] Kim J, Johnson M, Hill P, Gale BK, Microfluidic sample preparation: cell lysis and nucleic acid purification. Integrative Biology 2009, 1(10):574-86. DOI: https://doi.org/10.1039/b905844c.
[60] Choi Y, Kim YT, Lee SJ et al., Direct Solvent-Free Modification of the Inner Wall of the Microchip for Rapid DNA Extraction with Enhanced Capturing Efficiency. Macromol. Res 2020, 28:249–256. DOI: https://doi.org/10.1007/s13233-020-8028-x.
Rights and Permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party materials in this article are included in the articles Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Creative Commons This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. You are not required to obtain permission to reuse this article. To request permission for a type of use not listed, please contact Biotechnology Kiosk.