The Biomedical Use of Radioisotopes of Thallium: A Potential Gateway to the Diagnosis of Chronic Diseases
Rajeev Gupta and D.P. Singh*
Department of Physics, School of Engineering Studies, University of Petroleum & Energy Studies, Dehradun-248007, Uttarakhand, India.
Abstract
Nuclear medicine is a specialized area of radiology that uses very small amounts of radioactive materials, or radiopharmaceuticals inside the body to examine organ function and structure for diagnosis or to target and destroy damaged or diseased organs or tissue (for treatment). Several different types of radionuclides are available. These include forms of the element technetium, thallium, gallium, iodine, and xenon. Out of these radionuclides, thallium’s radioisotope plays a crucial role in the diagnosis of chronic ailments like CAD, myocardial infarction, ischemia, to name few. These diseases cause specific muscles or arteries to die or get blocked. Out of the radioisotopes of thallium, Tl-201mis is considered best suited for diagnosis of such diseases by tomography techniques such as Single Photon Emission Computed Tomography (SPECT). In this article, we have presented an overview of how thallium can be used as a nuclear medicine to test the stress level in human, mechanism of action as a tracer and perfusion imaging test by using SPECT.
Keywords: Radioisotopes, Nuclear Reaction, Nuclear Medicine, chronic diseases, SPECT
*Corresponding author:
Dr. D.P. Singh; +91 6397704311
dpsingh19@gmail.com
Received: August 5, 2021
Revised manuscript: August 20, 2021
Accepted: August 25, 2021
Introduction
Henri Becquerel discovered radioactivity while working with uranium salt in 1896, which led to the development and use of radioactive isotopes and nuclear physics[1]. After that, for about five decades, i.e., from the 1890s to 1940s, many scientists like Marie and Pierre Curie, Ernest Rutherford, and James Chadwick did extensive research. In the last few years of this era, there were significant developments in this field due to Nuclear Bombs used in WW2. Further discoveries and inventions led to energy production, and other uses like nuclear medicine came to light due to their decay properties[2]. Radioisotopes are used to detect cracks in heavy mechanical systems, leak detection in long pipelines, and detect nuclear activities from nuclear weapons[3]. The field of Nuclear Medicine had its breakthrough when Sam Seidlin 1946 reported successful treatment of a patient’s thyroid cancer[4]. Later in the 1950s, tracers’ diagnostic and imaging properties also started to get international attention. After years of research and development, we have various applications in this stream, like diagnoses and treatment of cancer, imaging technique in coronary artery disease (CAD), ischemia, myocardial infarction, and locating lymphoma[5].
Radioisotopes like Iodine 131, Strontium 89, Bismuth 212, Thallium 201, and others are used for radiotherapy, diagnosis, pain relief, etc. With increased uses and applications comes the problem of efficient, economical, and safe production of radioisotopes[6]. Because radioisotopes are either rare or must have a high level of energy to be produced, it is well known that most are very difficult to produce[7]. Hence, conducting real-time experiments for the above-stated reasons is not always feasible. Thallium-201 is a potentially helpful radioisotope for various medical applications, including myocardial visualization and possible physiology assessment, as a renal medullary imaging agent, and as tumor detection[8]. It is biologically similar to potassium in organ distribution and neurophysiologic function[9]. The physical-chemical explanation for the biological similarity of Tl+ and K+ is that the hydrated ionic radius of Tl+ is between K+ and Rb+ in size, and this radius has been suggested as the property that determines passive penetration through a membrane[10]. These facts indicate that radiothallium should be a good potassium analog and therefore has the potential for myocardial visualization and the early detection of areas of diminished perfusion and radionuclide uptake as cold spots (regions of decreased activity)[11].
Thallium as Nuclear Medicine
Thallium 201 is produced by bombarding the target of natural thallium by an external beam of protons in a cyclotron[12]. Thallium is generally used for diagnostic purposes, where it is used in its chloride form by intravenous administration. Some of the isotopes of thallium do not particularly have any medicinal or non-medicinal uses, Tl200 and Tl202 are usually eliminated from the mixture of nuclear medicine as it is difficult to excrete and makes the drug unstable.
Thallium acts as a radiopharmaceutical agent used for various purposes like the diapurposes purpose of CAD, parathyroid activity, ischemia, and lymphoma, etc. [13]. Thallium is a white/silvery metal that easily tarnishes and is a chemically toxic metal initially used as a rodenticide, but it later came to light with properties that enabled it to be used as a nuclear medicine tracer in the photoelectric cell thermometer, etc. [14].
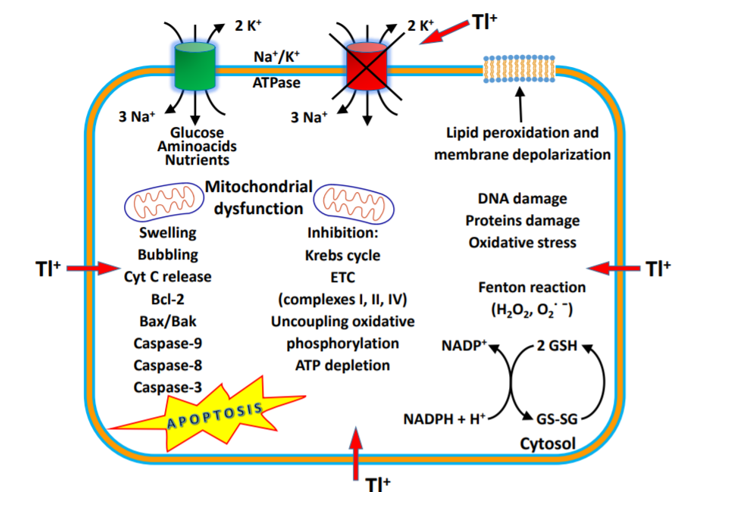
Figure 1. Mechanism of thallium action [Source: Journal of Head Trauma Rehabilitation (2016)].
Tl+ in different concentrations induced a significant increase in mitochondrial ROS formation, ATP depletion, glutathione oxidation, mitochondrial membrane potential (MMP) collapse and mitochondrial outer membrane rupture with cytochrome C release, and peroxidation of membrane phospholipids, especially binding to anionic head groups (Fig 1). This suggests that Tl+ may alter the fluidity of the mitochondrial membranes, acting on phospholipid packing, affecting the activities of membrane-associated transport systems and enzymes, and disrupting receptor functions[15].
Mechanism of action as a tracer
Thallium is used to test the stress level in humans and is called the cardiac or thallium stress test. It is a nuclear imaging test showing how well blood flows into your heart while exercising or resting[16]. In this, a liquid with a small amount of radioactivity called a radioisotope is administered into one of your veins. The radioisotope will flow through your bloodstream and end up in your heart. Once the radiation is in your heart, a special camera called a gamma camera can detect the radiation and reveal any issues your heart muscle is having[17]. The thallium test provides more information about heart chamber size, heart pumping efficiency, coronary artery supply to the heart, and perfusion of myocardial muscle after previous heart attacks [Fig 2]. At the same time, the thallium stress test has several risks and complications. Complications from the radioactive material injected into your body are infrequent, including arrhythmia or irregular heartbeat, increased angina, pain from poor blood flow in your heart, difficulty breathing, asthma-like symptoms, and skin rashes.
Initially, A stabilized chloride form of thallium is prepared for injection into veins, then injected into a patient’s body via intravenous injection. In about 5 to 15 minutes after injection, thorium-201 is taken up by the myocytes (the biological name for muscle cells or fibers). The Thallium 201 isotope acts as a tracer that travels through the bloodstream.
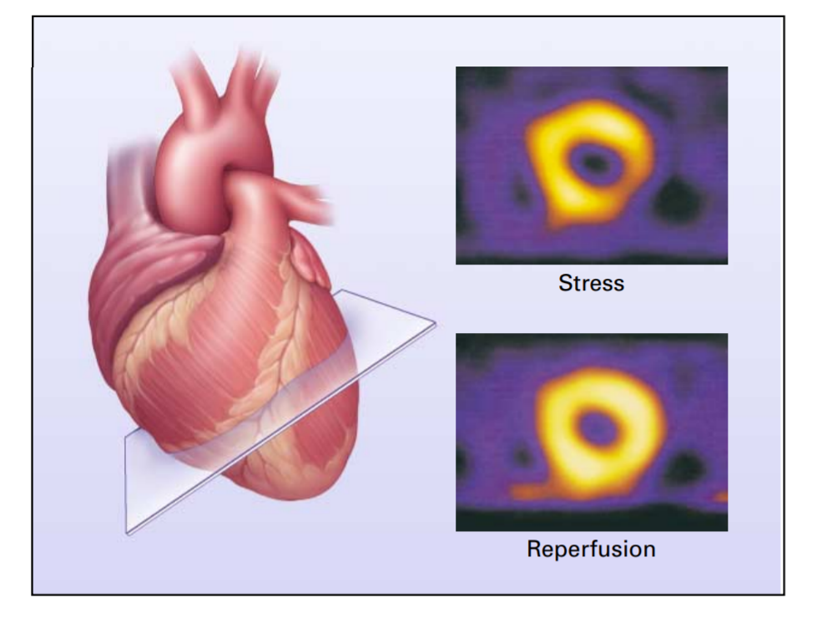
Figure 2: Use of Radionuclide Scintigraphy to Assess Myocardial Perfusion and Viability [Source: The New England Journal of Medicine (2001)].
Structurally, Tl 201 is similar to potassium; hence, it uses the Na-K pumps in the human body to get absorbed into cells. And then imaging, i.e., stress imaging, is done in two to three stages based on the time after injection, like stress test imaging 15 mins after injection, rest imaging about 2 to 4 hours after injection, and redistribution imaging 24 hours after injection. Contrary to normal cells, the ischemic cells show delayed uptake of the radioactive tracer is concluded by comparing the image from 3- staged imaging. The affected areas (dead myocytes or improper blood flow regions) do not absorb the tracer properly. These areas are called cold spots or defects and can be seen dark patches in the MPI scan images.
Thallium uses in Single Photon Emission Computed Tomography (SPECT) for perfusion imaging test
Thallium Stress Test is a type of nuclear scanning test or myocardial perfusion imaging test. Myocardial perfusion imaging (MPI) is a diagnostic procedure used to show how well your heart muscle is being supplied (perfused) with blood at rest and under stress (such as exercise). It shows areas of the heart that have reduced blood supply due to narrowing of one or more coronary arteries. A small amount of radioactive tracer is used to show the heart muscle. Areas of the heart receiving adequate blood flow are yellow on a monitor. Those with reduced flow are blue. SPECT is the best and oldest technique to access myocardial perfusion, and thallium-201, 99mTc-sestamibi, or 99mTc-tetrofosmin are frequently used tracers for regional perfusion. Perfusion defects may be fixed (identical perfusion defect at rest and stress images) or reversible (perfusion defect on stress images normalizes on rest images), representing myocardial scar or ischemia, respectively [Fig 3].
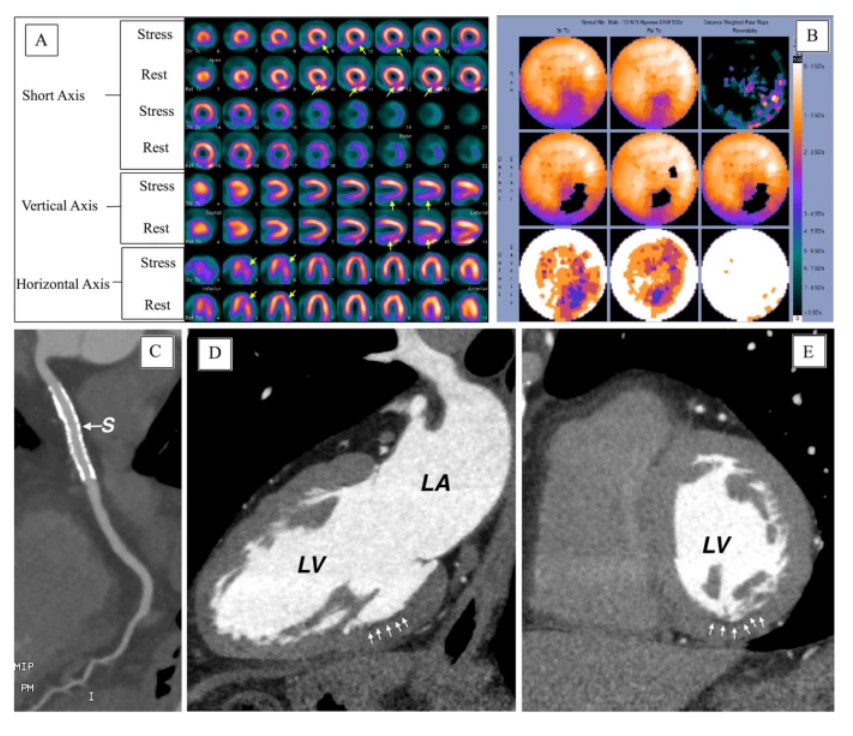
Figure 3. (A) SPECT images, (B) polar maps at stress (upper panel) and rest (lower panel), (C–E) computed tomographic angiography images (LV, left ventricle; LA, left atrium) [Source: European Journal of Hybrid Imaging (2022)].
In SPECT, evaluation of function is inferior to perfusion analysis. Still, post-ischemic stunning may be detectable in severely ischemic areas if ECG-gating is performed for stress image. In contrast, fixed perfusion defects are usually associated with regional wall motion abnormalities on gated rest images. Additional high-risk criteria on SPECT include transient ischemic dilation, increased lung uptake, reduced post-stress ejection fraction, and abnormal right ventricular myocardial uptake. The drawbacks of SPECT include a high-radiation dose (ranging from 10 mSv with 99mTc-labeled tracers to 25 mSv with thallium), limiting its use for serial follow-up, especially in young women. Additionally, obese subjects are susceptible to more photon attenuation artifacts and noninterpretable scans. Furthermore, myocardial perfusion SPECT does not provide absolute myocardial blood flow but only relative perfusion defects, i.e., ischemic territories, compared to remote myocardium. Due to this intrinsic limitation, SPECT may underestimate the extent of disease in multivessel disease with globally reduced myocardial perfusion. On the other hand, impaired renal function is never, and claustrophobia rarely, a contraindication for SPECT.
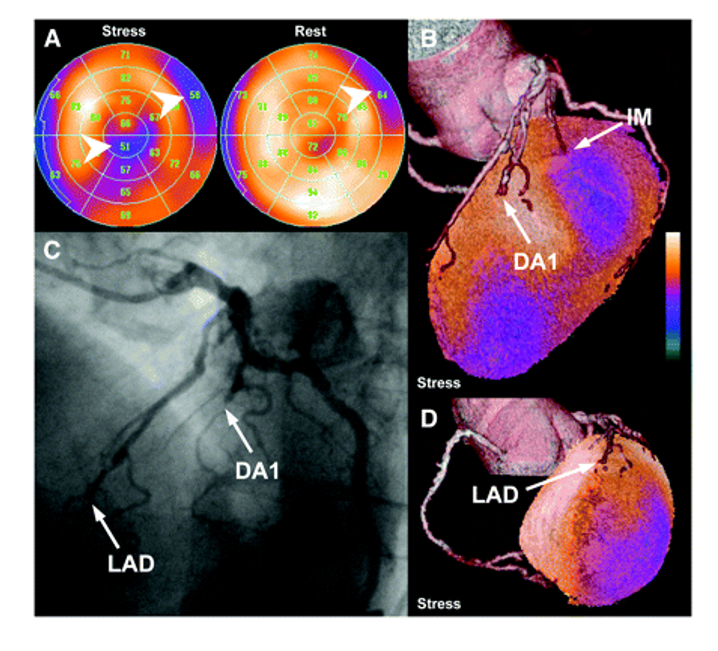
Figure 4: (A) Stress and rest perfusion polar maps of SPECT-MPI study show mixed basal anterolateral defect and reversible infer apical perfusion defect (arrowheads). (B and D) Fused SPECT/CT images reveal total occlusion of LAD and subtotal occlusion of first diagonal branch (DA1), which are confirmed by conventional CA (C). Anterolateral perfusion defect is caused by lesion of partially calcified small intermediary branch (IM); however, this vessel is not well visualized by CA [Source: Journal of Nuclear Medicine May (2007)].
Oliver[18] successfully performed the fusion of CTA and SPECT-MPI in all patients. In a side-by-side analysis of 40 equivocal coronary lesions, 14 showed a perfusion defect in their corresponding coronary artery territory, thus indicating hemodynamic relevance. The fused analysis, however, excluded hemodynamic significance in 10 coronary lesions. Despite the vessel being reliably identified, identifying the culprit lesion for the remaining 16 lesions remains inconclusive, even though eight pairs of consecutive neighboring lesions were present. According to the fused analysis of three lesions (hemodynamically relevant, n = 2; irrelevant, n = 1; patients 22 and 30), the assessment of perfusion defect and coronary lesion appeared trustworthy on side-by-side analysis but unreliable on side-by-side analysis (patients 22 and 30). This allowed for the exclusion of a relevant lesion in significantly more segments based on the fused SPECT/CT interpretation than the side-by-side analysis [Fig. 4]. A typical test result indicates that your heart has adequate and unrestricted blood flow when resting and exercising. When your heart works harder during strenuous activity, your blood flow may not be good during the exercise phase of your stress test[19]. There is likely some level of coronary artery disease or blockage during exercise, even if your blood flow is adequate at rest[20]. No matter how much exertion you put into your stress test, an abnormal result indicates poor blood flow in your heart[15]. Significant coronary artery disease is characterized by restricted blood flow. You may have scar tissue or damaged tissue caused by a previous heart attack if areas of the heart are not highlighted with the radioactive isotope on your stress test images.
Conclusion
We have described biomedical significance of using radiopharmaceuticals inside the body. This nuclear medicine provides a diagnostic tool for seeing how organs function and to detect damaged or diseased tissues or organs. Several different types of radionuclides like technetium, thallium, gallium, iodine, and xenon have been studied for use in diagnose chronic ailments such as CAD, myocardial infarction, ischemia, etc. These radionuclides have been shown helpful to prevent medical condition involving specific muscle or artery that gets blocked or die due to these diseases. The best use of radioisotope of thallium has been suggested in tomography techniques in diagnosing such diseases.
References-
[1] Radvanyi P., Villain J., The discovery of radioactivity, Comptes Rendus Phys. 2017; 18: 544–550. https://doi.org/10.1016/j.crhy.2017.10.008.
[2] Kalair A., Abas N., Saleem M.S., Kalair A.R., Khan N., Role of energy storage systems in energy transition from fossil fuels to renewables, Energy Storage. 2021; 3: e135. https://doi.org/10.1002/est2.135.
[3] Mehta V.K., McClure P., Kotlyar D., Selection of a Space Reactor Moderator Using Lessons Learned from SNAP and ANP Programs, in: AIAA Propuls. Energy 2019 Forum, American Institute of Aeronautics and Astronautics, Reston, Virginia, 2019; https://doi.org/10.2514/6.2019-4451.
[4] Croll M.N., Historic perspective, Semin. Nucl. Med. 1994; 24: 3–10. https://doi.org/10.1016/S0001-2998(05)80245-4.
[5] Hussain S., Mubeen I., Ullah N., Shah S.S.U.D., Khan B.A., Zahoor M., Ullah R., Khan F.A., Sultan M.A., Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review, Biomed Res. Int. 2022; 2022: 1–19. https://doi.org/10.1155/2022/5164970.
[6] Holik H.A., Ibrahim F.M., Elaine A.A., Putra B.D., Achmad A., Kartamihardja A.H.S., The Chemical Scaffold of Theranostic Radiopharmaceuticals: Radionuclide, Bifunctional Chelator, and Pharmacokinetics Modifying Linker, Molecules. 2022; 27: 3062. https://doi.org/10.3390/molecules27103062.
[7] Curry A., Bonsor A., Lichtenberg T., Shorttle O., Prevalence of short-lived radioactive isotopes across exoplanetary systems inferred from polluted white dwarfs, Mon. Not. R. Astron. Soc. 2022; 515: 395– 406. https://doi.org/10.1093/MNRAS/STAC1709.
[8] Alenezi S.A., Elgazzar A.H., Endocrine System, in: Pathophysiol. Basis Nucl. Med., Springer International Publishing, Cham, 2022: 219–261. https://doi.org/10.1007/978-3-030-96252-4_6.
[9] Fan S.-Y., Khuntia S., Ahn C.H., Zhang B., Tai L.-C., Electrochemical Devices to Monitor Ionic Analytes for Healthcare and Industrial Applications, Chemosensors. 2022; 10; 22. https://doi.org/10.3390/chemosensors10010022.
[10] Biondo F., Baldassarre F., Vergaro V., Ciccarella G., Controlled biocide release from smart delivery systems, in: Nanotechnology-Based Sustain. Altern. Manag. Plant Dis., Elsevier, 2022; 31–147. https://doi.org/10.1016/B978-0-12-823394-8.00010-X.
[11] Singh D., Kaur P., Attri S., Singh S., Sharma P., Mohana P., Kaur K., Kaur H., Singh G., Rashid F., Kumar A., Rajput A., Bedi N., Singh B., Buttar H.S., Arora S., Recent Advances in the Local Drug Delivery Systems for Improvement of Anticancer Therapy, Curr. Drug Deliv. 2021; 18.
https://doi.org/10.2174/1567201818666211214112710.
[12] Nath-M R., Simulating the Production of Medical Radioisotopes in a Fast Thorium-Cycle ADS with SERPENT, World J. Nucl. Sci. Technol. 2021; 11: 43–64. https://doi.org/10.4236/wjnst.2021.111003.
[13] Rigby A., Firth G., Rivas C., Pham T., Kim J., Phanopoulos A., Wharton L., Ingham A., Li L., Ma M.T., Orvig C., Blower P.J., Terry S.Y.A., Abbate V., Toward Bifunctional Chelators for Thallium-201 for Use in Nuclear Medicine, Bioconjug. Chem. 2022; 33: 1422–1436. https://doi.org/10.1021/acs.bioconjchem.2c00284.
[14] Zhong Q., Qi J., Liu J., Wang J., Lin K., Ouyang Q., Zhang X., Wei X., Xiao T., El-Naggar A., Rinklebe J., Thallium isotopic compositions as tracers in environmental studies: A review, Environ. Int. 2022; 162: 107148. https://doi.org/10.1016/j.envint.2022.107148.
[15] Clausen M., Pendergast D.R., Willer B., Leddy J., Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes, J. Head Trauma Rehabil. 2016; 31: 215–224. https://doi.org/10.1097/HTR.0000000000000145.
[16] Dilsizian V., Rocco T.P., Freedman N.M.T., Leon M.B., Bonow R.O., Enhanced Detection of Ischemic but Viable Myocardium by the Reinjection of Thallium after Stress-Redistribution Imaging, N. Engl. J. Med. 1990; 323: 141–146. https://doi.org/10.1056/NEJM199007193230301.
[17] Zierler K.L., Equations for Measuring Blood Flow by External Monitoring of Radioisotopes, Circ. Res. 1965; 16: 309–321. https://doi.org/10.1161/01.RES.16.4.309.
[18] Gaemperli O., Schepis T., Valenta I., Husmann L., Scheffel H., Duerst V., Eberli F.R., Luscher T.F., Alkadhi H., Kaufmann P.A., Cardiac Image Fusion from Stand-Alone SPECT and CT: Clinical Experience, J. Nucl. Med. 2007; 48: 696–703. https://doi.org/10.2967/jnumed.106.037606.
[19] SALTIN B., RÅDEGRAN G., KOSKOLOU M.D., ROACH R.C., Skeletal muscle blood flow in humans and its regulation during exercise, Acta Physiol. Scand. 1998; 162: 421–436. https://doi.org/10.1046/j.1365-201X.1998.0293e.x.
[20] Reffelmann T., Post-stenotic coronary blood flow at rest is not altered by therapeutic doses of the oral antidiabetic drug glibenclamide in patients with coronary artery disease, Heart. 2002; 87: 54–60. https://doi.org/10.1136/heart.87.1.54.