

Corresponding Author:
Dr. Govind Singh
Department of Pharmaceutical Sciences,
Maharshi Dayanand University,
Rohtak, Haryana, 124001, India
drgovind.pharma@mdurohtak.ac.in
ABSTRACT
Traumatib brain injury (TBI) is a primary public health concern that has caused millions of deaths and disabilities around the world. Numerous medications are available to relieve TBI-associatedcomplications. However, these medications do not prevent further harm from occurring. Therefore, the unmet need is the development of novel therapeutic medicines that protect against neuronal damage as a result of trauma and its repercussions, especially from secondary injury. We present a study using Swiss albino mice (25-30 g) of either sex to address the therapeutic issues concerning TBI. In our study, TBI was induced by the weight-drop method. Oxidative stress parameters were observed following the administration of zonisamide (100 mg/kg) and Nigella sativa(NS) (300 mg/kg) per se and in combination. The levels of glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) were observed to be significantly enhanced, but the levels of malondialdehyde(MDA) and nitric oxide (NO) were significantly reduced by treatment with the mentioned dugs. Our findings affirmed the potential function of both drugs in preventing TBI-induced oxidative damage.
KEYWORDS
Nigella sativa, Oxidative stress, Traumatic brain injury, Weight drop model, Zonisamide.
INTRODUCTION
Traumatic brain injury (TBI) is considered a global health threat [1] that kills and disables millions of people every year[2]. Estimated 64 to 74 million new TBI cases occur every year, with the highest prevalence in the United States, Canada, and Europe[3], with around 53,000 casualties and 283,000 hospitalizations. Furthermore, on a larger scale, the predicted existence of TBI in youngsters aged 0-14 is about 475,000 [4]. TBI also has a substantial financial cost to societies, with an estimated $400 billion healthcare cost that is spent annually to manage TBI [5].
One of the aspects that influences TBI outcomes are biochemical cascades that emerge following primary and secondary injury. TBI creates a metabolic and ionic imbalance, resulting in an overabundance of reactive oxygen species (ROS) production and oxidative stress.[6,7], leading to brain malfunction and mortality. After TBI, various oxidative stress markers, including reactive oxygen species (ROS), are formed in the brain. At the same time, the level of anti-oxidant enzymes decreases. As a result, developing anti-oxidant techniques are a top priority in current attempts to treat trauma-induced brain injuries. It is to be noted that the US food and drug administration (FDA) has yet to approve a medicine for TBI treatment[8].
Nonetheless, it is expected that focusing on the pathological mechanisms might help to mitigate TBI’s effects. Anti-oxidants have been investigated as a therapy option for TBI and have been demonstrated to have a neuroprotective impact. Excitatory amino acids like glutamate are released into the synapse after mechanical stress, overstimulating N-methyl-D-aspartate (NMDA) receptors. As a result, there is a Ca2+ overload and enhanced depolarization due to ionic imbalance. Excessive formation of ROS and, as an outcome, oxidative stress are caused by an excess of intracellular Ca2+ and excitotoxicity [9].
Zonisamide is a novel broad-spectrum antiepileptic drug that works well for refractory partial seizures[10–12]. Zonisamide has a structural, molecular, and pharmacokinetic profile apart from other antiepileptic drugs[10]. Zonisamide is considered to help dopaminergic and serotonergic neurotransmission by blocking voltage-dependent Na+ channels, lowering voltage-dependent T-type inward Ca2+ currents, binding to the GABA–benzodiazepine receptor complex, and blocking voltage-dependent Na+ channels. Zonisamide reduces Ca2+-dependent K+ evoked extracellular glutamate release[14,15].
Recently, plants and herbs’ low toxicity and cost-effectiveness have gained attention to investigate potential pharmacological activities. Nigella sativa (NS) is an herb belonging to the family Ranunculaceae, and its seeds are conventionally used to treat various diseases around the globe[16]. NS and its active component, thymoquinone have been shown in several human and animal investigations to have antioxidative properties[17]. Immunomodulatory[18], neuroprotective[19], antibacterial[20], hypertensive[21,22], and hypoglycemic[23]effects. The protective effect of NS to demolish oxidative stress plays a pivotal role in treating various diseases such as cancer, arteriosclerosis, and ischemia, in which free radical generation is involved[24,25]. The usage of NS extract and thymoquinone also results in morphologic improvement and apoptosis, thus suggesting NS therapy could be helpful in the prevention of neurodegeneration.
We have presented an animal study on neuroprotective potential of Zonisamide and Nigella sativa on traumatic brain injury-induced oxidative stress.
MATERIALS AND METHODS
Animal
The study used Swiss albino mice (25-30 g) of either sex, kept under natural day and night cycles in polypropylene cages with rodent food and water ad libitum. The Institutional Animal Ethics Committee of Maharshi Dayanand University Rohtak, Haryana, India, authorized all experimental protocols.
Induction of TBI by weight drop method
Mice were given a 2% isoflurane anaesthetic and permitted to breathe normally without tracheal intubation. After that, mice were placed on a sponge using surgical tape, and a small longitudinal incision was given to the overhead of mice exposed to the skull. The metallic disc was centrally fixed on the exposed skull and adequately placed the mice under the metallic pipe. Then the metallic spherical weight (60 g) freely falls through the metallic pipe over the head of the mice. After that metallic disc was removed, the exposed skull was sutured. Finally, neosporin powder was spread over the surgery site and returned to its home case for recovery.
Treatment schedule
The experimental study was designed to determine oxidative parameters at 3, 6, 24, and 72h. The zonisamide and NS were administered per se and in combination 30 min after the induction of TBI. Mice were divided into five groups, each consisting of 6 mice. Group 1, the control group, did not receive any injury or drug treatment, whereas TBI was induced in all other four groups then Group 2 receivedvehicle, Group 3 receivedzonisamide (100 mg/kg), Group 4 received NS (300 mg/kg) and Group 5 received combination of both zonisamide (100 mg/kg) and NS (300 mg/kg).
Oxidative parameters
Tissue preparation: After cervical dislocation at 3, 6, 24, and 72h, the animal’s brain was removed quickly and dipped in ice-cold normal saline (0.9% NaCl); after that, brain tissue was homogenized immediately in an ice-cold pH 7.4 phosphate buffer with 10 times its weight. The brain homogenates were centrifuged for 15 minutes at 3000 rpm. An aliquot of supernatants and cell pellets was separated and kept at -20°C in deep freeze (Blue Star Pvt. Ltd., India) until further oxidative parameters estimations were processed.
Superoxide dismutase (SOD) estimation: In this method, 0.15mL of hydroxylamine hydrochloride was added to a reaction mixture containing 75µl nitro blue tetrazolium (NBT), 1.9mL distilled water, 0.15mL Triton-X100, and 0.1mL supernatant. The inhibition of reduction of NBT by superoxide dismutase (SOD) present in the supernatant was then estimated by measuring the absorbance of the mixture at560nm using a UV–visible spectrophotometer (Shimadzu UV-1800) against a blank, and The concentration of SOD was measured as unit/min/mg of protein[26].
Catalase (CAT) estimation: 0.1mL brain supernatant was combined with 0.1mL phosphate buffer (0.01M, pH 7.4) and 0.4mL distilled water. Then 0.5mL solution of 2M hydrogen peroxide was added to start the reaction. The potassium dichromate acetic acid reagent was later added in a volume of 2mL, and after 15 minutes in a boiling water bath, the reaction was allowed to cool. The green color solution was then measured at 570 nm using a UV–visible spectrophotometer against a blank, and CAT concentration was expressed as µmol/mg protein[27].
Malondialdehyde (MDA) estimation: In this method, 0.1 mL of the supernatant was mixed with 1.5 mL of acetic acid (20%, pH 3.5), 1.5 mL of thiobarbituric acid (0.8%), and 0.2 mL of sodium lauryl sulfate (8.1%). After that, the mixture was heated for 60 min. at 100°C and chilled with running water. After that 5mL of n-butanol-pyridine (15:1v/v) and 1mL of distilled water were added. It was shaken energetically for 10 min, then centrifuged at 4000 rpm. The pink color organic layer produced was measured at 532nm using spectrophotometer against a blank solution. The results were expressed as nmol/mg protein[28].
Measurement of reduced glutathione (GSH): The processed sample was mixed with an equal volume of 10% trichloroacetic acid. At 4°C, the sample was centrifuged for 10 minutes at 2000 rpm. 0.1 mL supernatant, 2 mL pH 8.4 buffer, 0.5 mL 5-nitrobenzoic acid, and 0.4 mL water were added to the mixture. At 412nm after 15 minutes, the absorbance was then measured, and the results were expressed as µg/mg protein[29].
Measurement of nitric oxide (NO) content: The supernatant (1mL) was combined with equivalent measurements of Greiss reagent (0.1 % sulphanilamide in 2.5 % phosphoric acid and 0.1percent N-(1-naphthyl)ethylenediaminedihydrochloride) and left at room temperature for 10min, and the absorbance was measured at 540 nm against a blank sample using a spectrophotometer and NO content was expressed as µmol/mg protein[30].
Statistical analysis:
One-way ANOVA was used to examine the data, followed by Bonferroni’s multiple comparison post hoc test. Data were denoted as mean ±SEM and statistical significance denoted as *, **, or *** for p <0.05, 0.01 and 0.001, respectively. p values were shown with ‘#’ representing p < 0.05 when compared with vehicle.
Results
Effect of zonisamide and NS on the activity of SOD
The level of SOD was observed at 3,6,24, and 72h, and the results are described in Figures 1(a), 1(b), 1(c), and 1(d), respectively. The level of SOD significantly improved zonisamide (100mg/kg) and NS (300mg/kg) compared to the vehicle-treated group at different time intervals. Still, the combination showed a more significant amplification in the SOD level. Although, at 72h, the SOD level of the co-administered group showed no significant difference compared to the control group.
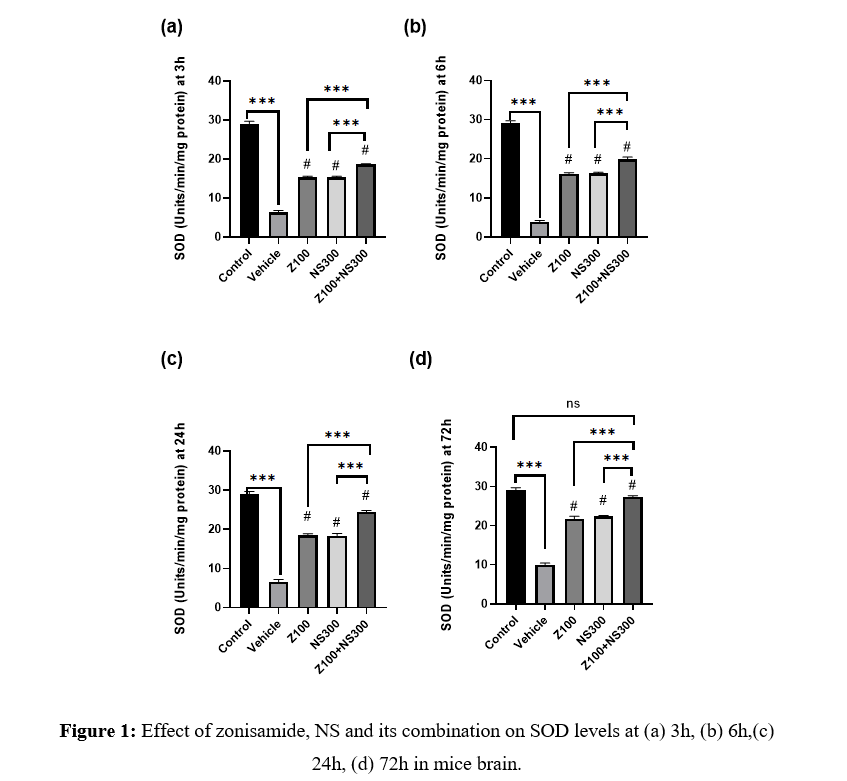
Effect of zonisamide and NS on Catalase level
The level of CAT was observed at 3,6,24 and 72h, and the result is described in Figures 2(a), 2(b), 2(c), and 2(d), respectively. The level of CAT significantly improved in zonisamide (100mg/kg) and NS (300mg/kg) compared to the vehicle-treated group at different time intervals. Still, the combination showed a more significant amplification in the CAT level. Although, at 72h, in compared to the control group, the co-administered group’s CAT level exhibited no significant difference.
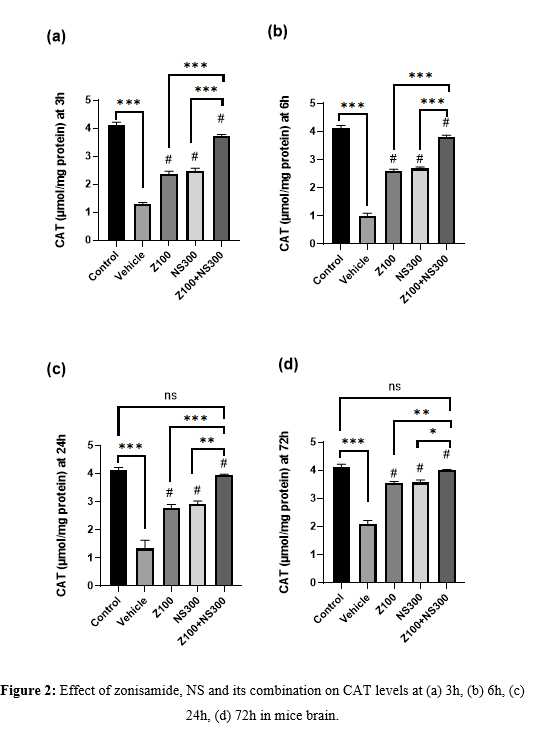
Effect of zonisamide and NS on MDA level
The level of MDA was observed at 3,6,24 and 72h, and the result is described in Figures 3(a), 3(b), 3(c), and 3(d), respectively. The MDA level was drastically increased in the vehicle-treated group. However, co-administration of both zonisamide (100mg/kg) and NS (300 mg/kg) showed a more considerable reduction in the MDA level than in vehicle-treated groups.
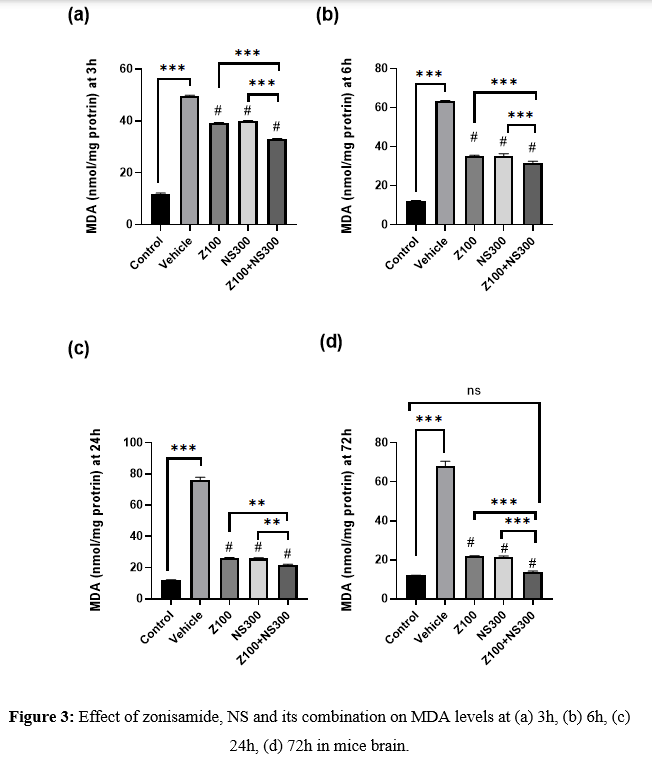
Effect of zonisamide and NS on GSH level
The level of GSH was observed at 3,6,24 and 72h, and the result is described in Figures 4(a), 4(b), 4(c), and 4(d), respectively. The GSH level significantly diminished in the vehicle compared to the control. On the other hand, the administration of zonisamide (100mg/kg) and NS(300mg/kg) extract per se remarkably increased the level of GSH. Still, the combination exhibited a more significant improvement in the GSH level than the vehicle-treated group at all time intervals. Although, at 72h, the GSH level of the co-administered group showed no remarkable difference compared to the control group.
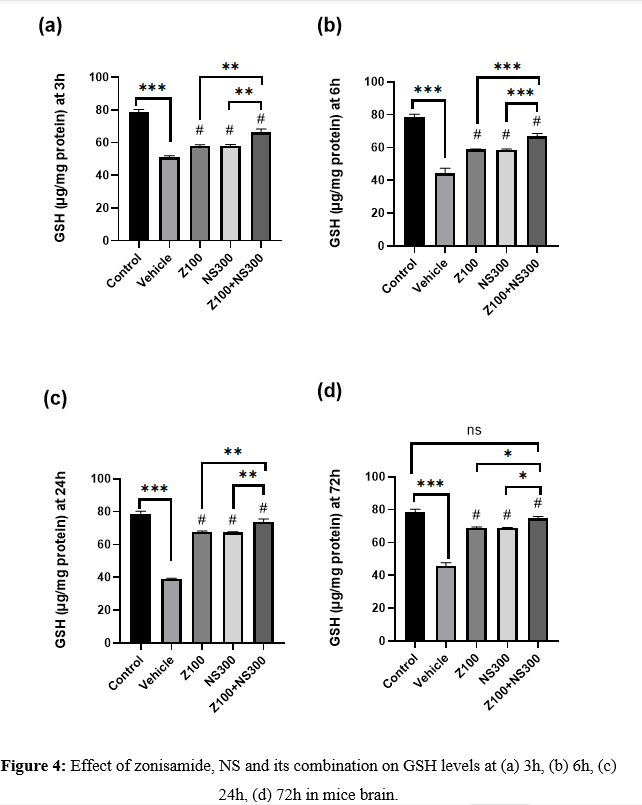
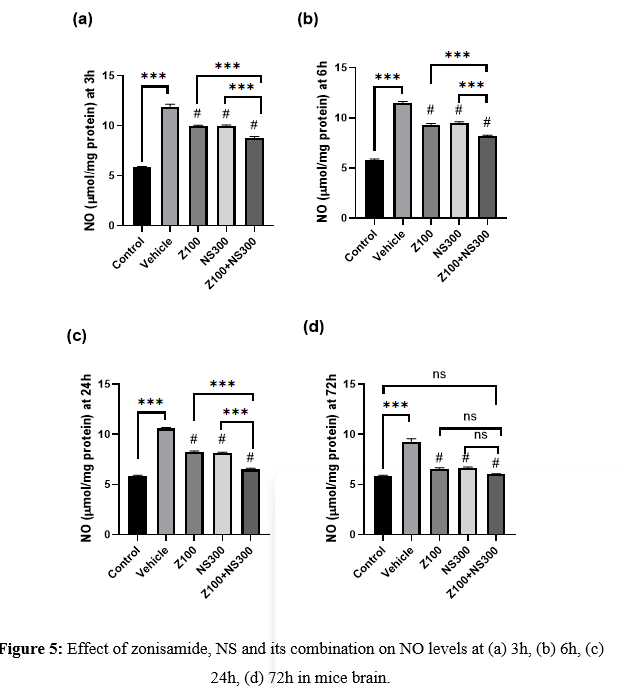
Effect of zonisamide and NS on NO level
The level of NO was observed at 3,6,24 and 72h, and the result is described in Figures 5(a), 5(b), 5(c), and 5(d), respectively. The level of NO significantly elevated after the injury, but the treatment with zonisamide and NS extract significantly decreased its levels. However, the co-administration of both zonisamide (100mg/kg) and NS (300mg/kg) demonstrated a more considerable MDA reduction than vehicle-treated groups. Although, at 72h, the NO level of in comparison to the control group, the co-administered group showed no significant differences.
DISCUSSION
TBI is still a primary clinical and socioeconomic concern, with few therapeutic options tailored to its clinical and neurobiological trajectory. The pharmacological potential of zonisamide and NS alone and in combination with a weight drop model against TBI in mice was explored in the present experiment. The goal of anti-oxidant neuroprotective drug development for acute TBI has always been to stop the subsequent injury cascade by pharmacologically addressing the oxidative damage mechanism. Oxidative stress occurs when the scavenging anti-oxidant system is overwhelmed by oxygen levels and oxygen-derived free radicals. On the other hand, the neuronal loss after hours and days is driven by secondary injury from inflammation, excitotoxicity of neurotransmitters, cerebral ischemia, blood-brain barrier disruption, and neurological deficits[31,32]. One of the essential aspects of TBI’s pathophysiology is oxidative stress. Many studies confirm that traumatic brain injury directly amplifies oxidative stress[33]. In the current investigation, the vehicle-treated group displayed more oxidative stress. Intervention with zonisamide (100mg/kg) and NS (300mg/kg) per se and their combination eliminate that oxidative stress by augmenting the level of SOD, CAT, and GSH. Still, the levels of NO and MDA significantly decrease. Zonisamide and NS reduced oxidative stress, resulting in neuroprotection by preventing secondary injury. Additionally, neuroprotection is more significant in the co-administered group, which could be attributable to many targets in the pathophysiological cascade of secondary damage following TBI.
CONCLUSION
According to the above observations, it was concluded that co-administration of zonisamide and NS revealed neuroprotective effects in mice against TBI-induced neurodegeneration by reducing oxidative stress. These anti-oxidant and neuroprotective properties could aid in developing these medications as a therapy for neuronal death caused by traumatic injuries.
REFERENCE
[1] Somayaji MR, Przekwas AJ, Gupta RK. Combination therapy for multi-target manipulation of secondary brain injury mechanisms. Current Neuropharmacology 2018;16:484–504. https://doi.org/10.2174/1570159X15666170828165711.
[2] Cash A, Theus MH. Mechanisms of Blood–Brain Barrier Dysfunction in Traumatic Brain Injury. International Journal of Molecular Sciences 2020;21:3344. https://doi.org/10.3390/ijms21093344.
[3] Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, et al. Estimating the global incidence of traumatic brain injury. Journal of Neurosurgery 2019;130:1080–97. https://doi.org/10.3171/2017.10.JNS17352.
[4] Postolache TT, Wadhawan A, Can A, Lowry CA, Woodbury M, Makkar H, et al. Inflammation in traumatic brain injury. Journal of Alzheimer’s Disease 2020;74:1–28. https://doi.org/10.3233/JAD-191150.
[5] Maas AIR, Menon DK, David Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017;16:987–1048. https://doi.org/10.1016/S1474-4422(17)30371-X.
[6] Shi K, Zhang J, Dong J, Shi F-D. Dissemination of brain inflammation in traumatic brain injury. Cellular & Molecular Immunology 2019;16:523–30. https://doi.org/10.1038/s41423-019-0213-5.
[7] Eakin K, Li Y, Chiang Y-H, Hoffer BJ, Rosenheim H. Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PLoS ONE 2013;8:82016. https://doi.org/10.1371/journal.pone.0082016.
[8] Ismail H, Shakkour Z, Tabet M, Abdelhady S, Kobaisi A, Abedi R, et al. Traumatic Brain Injury: Oxidative Stress and Novel Anti-Oxidants Such as Mitoquinone and Edaravone. Antioxidants (Basel) 2020;9:1–18. https://doi.org/10.3390/ANTIOX9100943.
[9] Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium 2004;36:257–64. https://doi.org/10.1016/J.CECA.2004.02.012.
[10] Schmidt D, Jacob R, Loiseau P, Deisenhammer E, Klinger D, Despland A, et al. Zonisamide for add-on treatment of refractory partial epilepsy: a European double-blind trial. Epilepsy Res 1993;15:67–73. https://doi.org/10.1016/0920-1211(93)90011-U.
[11] Faught E, Ayala R, Montouris GG, Leppik IE. Randomized controlled trial of zonisamide for the treatment of refractory partial-onset seizures. Neurology 2001;57:1774–9. https://doi.org/10.1212/WNL.57.10.1774.
[12] Sackellares JC, Ramsay E, Wilder BJ, Iii RB, Shellenberger K. Randomized, controlled clinical trial of zonisamide as adjunctive treatment for refractory partial seizures. Epilepsia2004;45:610–7.
[13] Seino M. Review of zonisamide development in Japan. Seizure 2004;13:S2–4. https://doi.org/10.1016/j.seizure.2004.04.015.
[14] Okada M, Kawata Y, Mizuno K, Wada K, Kondo T, Kaneko S. Interaction between Ca2+ , K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. British Journal of Pharmacology 1998;124:1277–85. https://doi.org/10.1038/sj.bjp.0701941.
[15] Matar N, Jin W, Wrubel H, Hescheler J, Schneider T, Weiergräber M. Zonisamide block of cloned human T-type voltage-gated calcium channels. Epilepsy Research 2009;83:224–34.
[16] Yimer EM, Tuem KB, Karim A, Ur-Rehman N, Anwar F. Nigella sativa L. (Black Cumin): A promising natural remedy for wide range of illnesses. Evidence-Based Complementary and Alternative Medicine 2019;2019:1–16. https://doi.org/10.1155/2019/1528635.
[17] Kanter M, Coskun O, Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Archives of Toxicology 2006;80:217–24. https://doi.org/10.1007/S00204-005-0037-1.
[18] Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytotherapy Research 2003;17:299–305. https://doi.org/10.1002/PTR.1309.
[19] Hobbenaghi R, Javanbakht J, Sadeghzadeh S, Kheradmand D, Abdi FS, Jaberi MH, et al. Neuroprotective effects of Nigella sativa extract on cell death in hippocampal neurons following experimental global cerebral ischemia-reperfusion injury in rats. J Neurol Sci 2014;337:74–9. https://doi.org/10.1016/J.JNS.2013.11.019.
[20] Chaudhry H, Fatima N, Ahmad IZ. Evaluation of Anti-oxidant and Antibacterial Potentials of Nigella sativa L. Suspension Cultures under Elicitation. BioMed Research International 2015;2015:1–13. https://doi.org/10.1155/2015/708691.
[21] Zaoui A, Cherrah Y, Alaoui K, Mahassine N, Amarouch H, Hassar M. Effects of Nigella sativa fixed oil on blood homeostasis in rat. Journal of Ethnopharmacology 2002;79:23–6. https://doi.org/10.1016/S0378-8741(01)00342-7.
[22] Jaarin K, Foong W, Yeoh M, Kamarul Z, Qodriyah H, Azman A, et al. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics 2015;70:751–7. https://doi.org/10.6061/clinics/2015(11)07.
[23] Meral I, Donmez N, Baydas B, Belge F, Kanter & M. Effect of Nigella sativa L. on heart rate and some haematological values of alloxan-induced diabetic rabbits. Scandinavian Journal of Laboratory Animal Science 2004;31:49–53. https://doi.org/https://doi.org/10.23675/sjlas.v31i1.59.
[24] el Tahir KEH, Ashour MMS, Al-Harbi MM. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: elucidation of the mechanism of action. General Pharmacology: The Vascular System 1993;24:1123–31. https://doi.org/10.1016/0306-3623(93)90359-6.
[25] Alhazmi MI, Hasan TN, Shafi G, Al-Assaf AH, Alfawaz MA, Alshatwi AA. Roles of p53 and Caspases in Induction of Apoptosis in MCF-7 Breast Cancer Cells Treated with a Methanolic Extract of Nigella Sativa Seeds. Asian Pacific Journal of Cancer Prevention 2014;15:9655–60. https://doi.org/10.7314/APJCP.2014.15.22.9655.
[26] Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch BiochemBiophys1978;186:189–95. https://doi.org/10.1016/0003-9861(78)90479-4.
[27] Chandra J, Mahadimane P V, Saqib A, Sukumaran S, Chandra S. Barium Chloride impairs physiology and brain glutamate in Cirrhinusmrigala during a short period of interaction. Egyptian Journal of Aquatic Biology & Fisheries 2020;24:995–1003.
[28] Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem1979;95:351–8. https://doi.org/10.1016/0003-2697(79)90738-3.
[29] Ellman GL. Tissue sulfhydryl groups. Arch BiochemBiophys1959;82:70–7. https://doi.org/10.1016/0003-9861(59)90090-6.
[30] Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem1982;126:131–8. https://doi.org/10.1016/0003-2697(82)90118-X.
[31] Xiong C, Hanafy S, Chan V, Hu ZJ, Sutton M, Escobar M, et al. Comorbidity in adults with traumatic brain injury and all-cause mortality: a systematic review. BMJ Open 2019;9:e029072. https://doi.org/10.1136/bmjopen-2019-029072.
[32] Larsen LK, Møller K, Petersen M, Egerod I. Delirium prevalence and prevention in patients with acute brain injury: A prospective before-and-after intervention study. Intensive and Critical Care Nursing 2020;59:102816. https://doi.org/10.1016/j.iccn.2020.102816.
[33] Rana A, Singh S, Deshmukh R, Kumar A. Pharmacological potential of tocopherol and doxycycline against traumatic brain injury-induced cognitive/motor impairment in rats. Brain Injury 2020;34:1039–50. https://doi.org/10.1080/02699052.2020.1772508.