Sequence polymorphism within erythrocyte binding domain of EBA175 in Indian and African field isolates
Monal Sharma1*, Shailja Singh2
International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India
Abstract
Invasion of erythrocyte by Plasmodium merozoites is mediated by specific molecular interactions between proteins expressed on merozoite surface and the receptors present on erythrocytes. Erythrocyte binding antigen 175 (EBA175) is one such protein that interacts with the sialic acid residues on glycophorin A present on erythrocytes’ surface during invasion. The FII region (PfFII) of EBA175 has been mapped to be critical for binding to erythrocytes. It is reported that antibodies against FII region blocks binding. Polymorphisms in FII region of EBA175 are already reported. The goal of this study was to investigate whether polymorphism in FII region of African P. falciparum field isolates has any effect on erythrocyte binding and also to find whether antibodies raised against FII region from P. falciparum Malayan Camp strain (Camp) can inhibit erythrocyte binding. Genomic DNA of parasites from the blood samples of P. falciparum infected individuals was isolated and PfFII region from these genomic DNA were amplified, cloned and sequenced. Following sequence analysis we selected three isolates harboring higher PfFII polymorphisms, expressed them on the surface of COS cells as chimeric proteins using secretory signal and transmembrane segments of Herpex simplex virus glycoprotein D (HSVg D) and tested for their erythrocyte binding ability. We further tested the inhibition of erythrocyte binding of these polymorphic FII regions using anti-campPfF2 antibodies. Our results reveal that the polymorphisms in different field isolates included in this study do not have any significant effect on erythrocyte binding and antibodies raised against FII region of camp strain could inhibit erythrocyte binding by all the polymorphic PfFII. This observation strengthens the possibility that PfFII can be a potential candidate vaccine.
1Current affiliation: Betterhumans, Inc., 3653 NE 77 Ave, Gainesville, FL 32609, USA
2Current affiliation: Jawaharlal Nehru University, New Delhi, India
*corresponding author: Dr. Monal Sharma
E-mail address: mons2179@gmail.com
Article type: Research article
Received: Nov 21, 2021
Revised: Dec 15, 2021
Accepted: Dec 20, 2021
Please cite this article as: Sharma M, Sequence polymorphism within erythrocyte binding domain of EBA175 in Indian and African field isolates, Biotechnol. kiosk, Vol 4, Issue 1, PP: 1-9 (2021); DOI: https://doi.org/10.37756/bk.22.4.1.1
Introduction
Invasion of erythrocyte by Plasmodium merozoites is mediated by specific molecular interactions between molecules expressed on merozoite surface and the receptors present on erythrocytes [1-3]. Erythrocyte binding antigen 175 (EBA-175) of Plasmodium falciparum is one such merozoite surface protein that interacts with the sialic acid residues on glycophorin A present on erythrocytes’ surface during invasion [4-6]. EBA-175 is localized in the micronemes at the apical end of the merozoites [7]. The gene encoding EBA-175 consists of an N terminal signal sequence, a long extracellular hydrophilic domain, a transmembrane sequence and a short C terminal cytoplasmic domain [8]. The extracellular domain contains 5’ and 3’ cysteine rich regions. The 5’ cysteine rich region contains two copies named FI and FII [9]. EBA-175 belongs to Duffy binding like erythrocyte binding protein family, because it shares sequence homology with members of this family [10]. The FII region (PfFII) of EBA-175 has been shown to be critical for binding to erythrocytes [11]. The antibodies against FII region block interaction between PfFII and erytrocytes-suggesting that PfFII is a potential vaccine candidate [10]. We investigated polymorphisms in FII region of African P. falciparum field isolates by PCR and found that antibodies raised against FII region from the P. falciparum Malayan Camp strain can inhibit erythrocyte binding by the field isolates having polymorphism.
Methods
DNA Isolation form filter paper
Genomic DNA of African P. falciparum field isolates was extracted from filter paper using the methanol fixation and heat extraction method [12]. Briefly, two-three pieces of filter paper was incubated with 500 μl of methanol for 15 min at room temperature. The methanol was drained off and the papers heated at 95-100°C in 50 μl of sterile distilled water for 15 minutes of incubation with intermittent vortexing. The samples were centrifuged and the final supernatant used as a template for the amplification reaction.
PCR amplification and sequence analysis of FII region of P. falciparum
Nested PCR technique was used to amplify gene fragment, which encodes for FII region of P. falciparum as decribed previously [12]. Briefly, for primary PCR reaction, primers EBARIIF (5’ggaagaaatacttcatctaataacg 3’) and EBARIIR (5’cgaagtttgttcattatttcttattatag 3’) were used and PCR product of primary PCR reaction was used as template for nested PCR with primers EBARIIF2 (5’ ttgatttagatgatttttctaaatttg 3’) and EBARIIR. The amplified PCR products were resolved on agarose gel (1.0%). PCR product was gel purified, ligated in pGEMT-E vector (Promega) and transformed in E.coli DH10B cells by CaCl2 heat shock method. Two clones of each field isolates, containing inserts were sequenced with T7 promoter, M13R and an internal primer by the cycle sequencing method using Big dye terminator kit (Applied Biosystem) and an ABI prism 310 automated DNA sequencer (Applied Biosystem). Analysis of sequence of FII region of field isolates was done using DNA- Star.
MSP typing of field isolates
PCR typing of these field isolates was done using a highly polymorphism markers, MSP1 and MSP2 as described in [14-15]. The Multiplicity of infection was estimated by the number of PCR fragments per infected individual.
Erythrocyte binding assay
To study the impact of point mutations on binding specificity of region II of P. falciparum field isolates, we chose three field isolates, which harbored more mutations in comparison to Indian field isolates. SE011/2, C032/5, C034/2 have seven, four and seven mutations respectively. These mutants and EBA-175 from CAMP strain were expressed on the surface of COS cells as chimeric proteins using secretory signal and transmembrane segments of Herpex simplex virus glycoprotein D (HSVg D) as described by [7, 16-17]. Surface expression of the chimeric protein on COS cells was determined by immuno-fluorescence using mAb DL6 against proline rich region of HSVgD and anti-mouse Ig-G labeled with FITC (Sigma). Transfected COS cells were incubated with untreated and enzyme (trypsin and neuraminidase) treated erythrocyte (10% hematocrit). Rosettes were scored in 20-25 fields at 40 X magnification.
Erythrocyte binding inhibition assay
Transfected COS cells were incubated with different dilutions (from 1:100 to 1:3200) of anti PfF2 antibodies for 1 hr at 370C and followed by 10% hematocrit. 10 –20 fields were scored for numbers of COS cells with adherent erythrocyte (rosettes) for control and test antibodies against region II of P. falciparum. In presence of different dilutions of anti-PfF2 antibodies, binding pattern was expressed relative to binding in presence of pre immune sera. Pre-immune sera (prior to immunization with PfF2) was used as a control at 1:100 dilution.
Results and Disussion
Diversity in PfFII region of field isolates
We observed 1 Kb amplified product in all field isolates. Region II was present in all field isolates of P. falciparum. Analysis of amino acid sequence of FII region from field isolates (Table 1) was showed that more than 98%. Sequence identity. All the cysteines and the positions of these cysteine residues in region II were also conserved. Another interesting observation was that all field isolates revealed polymorphisms at 15 amino acid positions when compared to the FII amino acid sequence of P. falciparum Malayan Camp and Indian field isolates. The present study identified 8 novel polymorphisms. In addition to the 8 novel polymorphisms, sequence diversity at seven positions namely 478, 481, 577, 584, 592, 664 and 716 were commonly present in African and Indian field isolates, which are already reported [18-19]. The non-conservative polymorphisms Asn (N) ↔ Lys (K), Ile (I) ↔ Lys (K), Lys (K) ↔ Gln (Q), Glu (E) ↔ Ala (A), Ser (S) ↔ Arg (R) were found in multiple isolates. And other polymorphisms were present in only one or two field isolates.
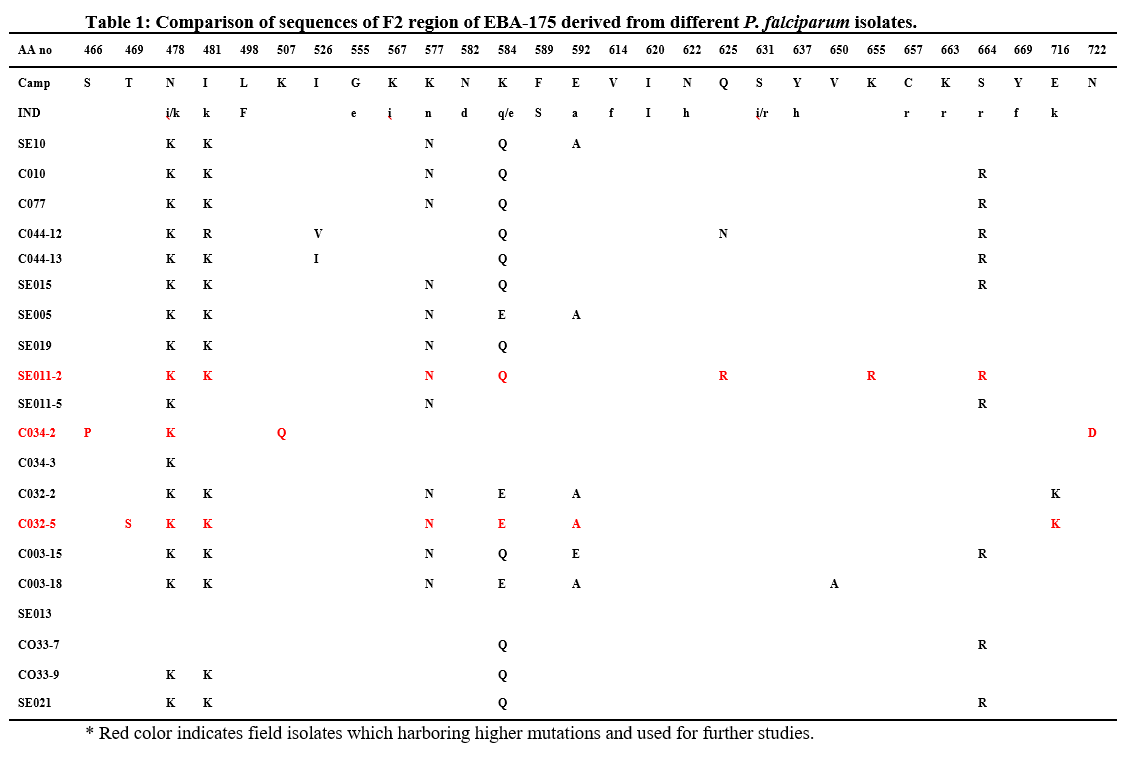
Allelic diversity in MSP typing
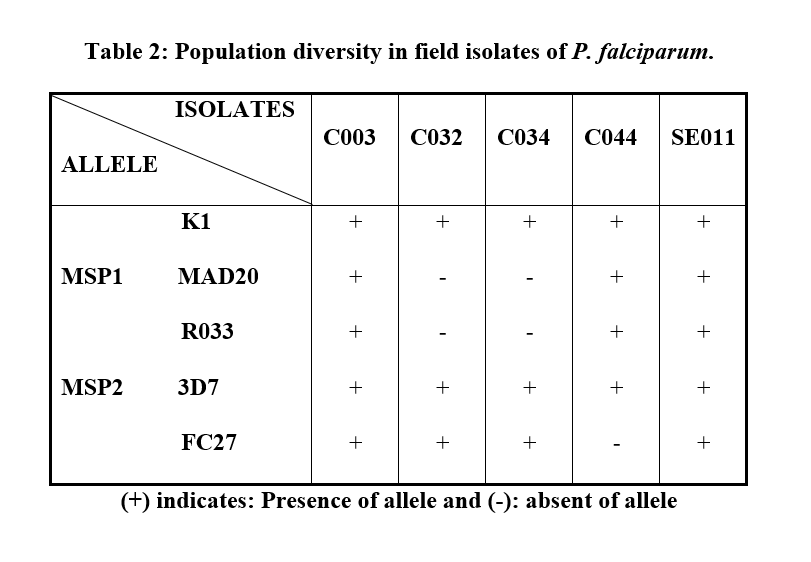
The population diversity was found in field isolates of P. falciparum namely C003, C032, C034, C044 and SE011. Three different pattern of genotype were observed (Table 2). K1 allele of MSP1 gene, 3D7 and FC27 alleles of MSP2 gene were observed in C032 and C034. C044 had K1, MAD20 and RO33 alleles of MSP1 and 3D7 allele of MSP2 gene. C003 and SE011 had K1, MAD20 and RO33 alleles of MSP1 gene and 3D7 and FC27 alleles of MSP2 gene. This suggested that the sampled patients had mixed infection.
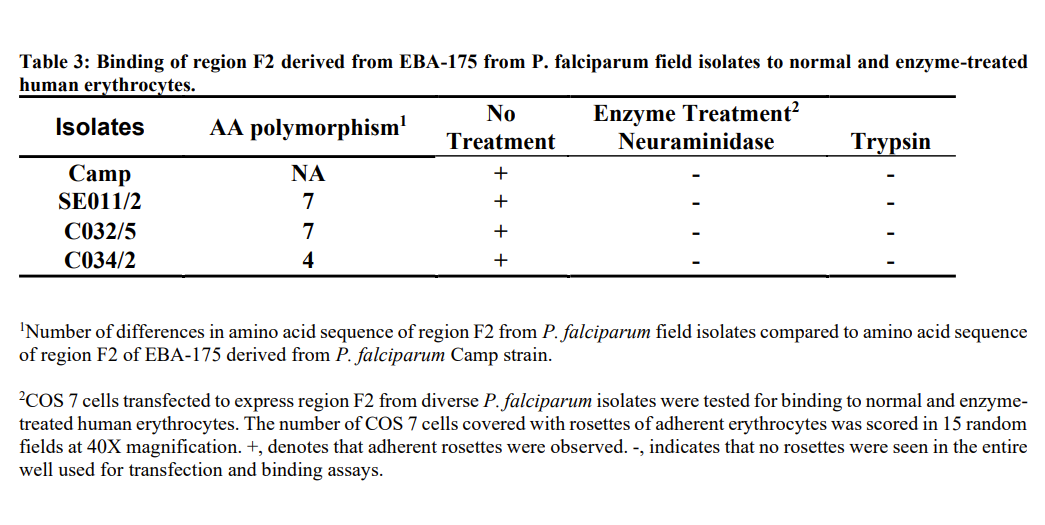
Erythrocyte binding of filed isolates
COS cells expressing region II bound normal human erythrocytes but not to enzyme (trypsin and neuraminidase) treated human erythrocytes (Table 3). The specificity of binding of normal and enzyme treated erythrocyte to region II expressed on COS cells was identical in native EBA-175 and P. falciparum field isolates [20]. From these experiments we conclude that region II of EBA-175 derived from P. falciparum field isolates binds erythrocyte with the same specificity as native EBA-175 despite the presence of polymorphic sequences.
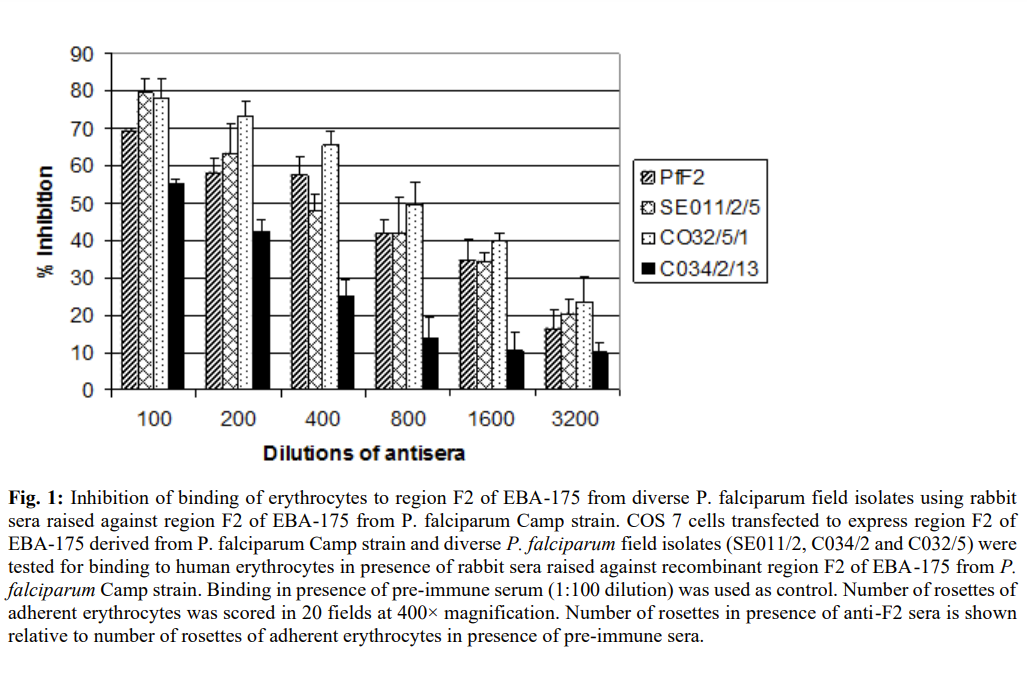
To evaluate the relative binding efficiency of variants, we measured inhibition of erythrocytes binding to region II expressed on COS cells with anti PfF2-antibodies. 70% inhibition was observed in all variants by anti-PfFII antibodies (figure 1). The result showed that antibodies against region II of P. falciparum inhibit invasion of erythrocytes in vitro and thus provides strong support for developing human vaccine based on region II of P.falciparum.
Conclusion
Erythrocyte binding antigen 175 (EBA-175) of Plasmodium falciparum belongs to Duffy binding like erythrocyte binding protein family. It contains three cysteine-rich regions F1, F2 and C in which F1 and F2 at the N-terminus are responsible for the glycophorin A binding on the erythrocyte membrane. The FII region (PfFII) of EBA-175 has been shown as a potential vaccine candidate. We investigated polymorphisms in FII region of African P. falciparum field isolates by PCR and found that antibodies raised against FII region from the P. falciparum Malayan Camp strain can inhibit erythrocyte binding by the field isolates having polymorphism. Our findings suggest that antibodies to region II of EBA-175 are largely unaffected by polymorphism in EBA-175 and is a good ligand-blocking malaria vaccine.
Authors’ contributions
Conceived and designed experiments: MS, SS, CEC. Performed the experiments: MS, and SS. Analyzed the data: MS, SS, CEC. Contributed reagents/materials/analysis tools: CEC. Wrote the paper: MS, SS and CEC. Involved in all the experiments: MS and SS.
Acknowledgement
This work has been funded by Program Support grant from DBT (CEC), MalSig and EVIMalaR grants from European Commission (CEC). SS is a recipient of the IYBA Award from DBT. CEC is a recipient of TATA Innovational Fellowship from DBT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
[1] Hardley TJ, Klotz FW, Miller LH: Invasion of erythrocytes by malaria parasites: a cellular and molecular overview. Annu Rev Microbiol 1986, 40:451-477.
[2] Cowman AF, Berry D, Baum J: The cellular and molecular basis for malaria parasite invasion of the human red blood cell. JCB 2012, 198: 961-971.
[3] Guar D, Chitnis CE: Molecular interactions and signaling mechanisms during erythrocyte invasion by malaria parasites. Curr opin Microbiol 2011, 14:422-428.
[4] Klotz FW, Orlandi PA, Reuter G, Cohen SJ, Haynes JD, Schauer R, Howard RJ, Palese P, Miller LH: Binding of Plasmodium falciparum 175-kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic acid but not its O-acetylated form. Mol Biochem Parasitol 1992, 51:49-54.
[5] Orlandi PA, Klotz FW, Haynes JD: A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac (alpha 2-3) Gal- sequences of glycophorin A. J Cell Biol 1992, 116:901-909.
[6] Ambroggio X, Jiang L, Aebig J, Obiakor H, Lukszo J, Narum DL: The Epitope of Monoclonal Antibodies Blocking Erythrocyte Invasion by Plasmodium falciparum Map to The Dimerization and Receptor Glycan Binding Sites of EBA-175. PLOS One 2013, 8: e56326.
[7] Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH: Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994, 264:1941-1944
[8] Adams JH, Kaneko O, Blair PL, Peterson DS: An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 2001, 17:297-299.
[9] Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH: A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci U. S. A. 1992, 89:7085-7089.
[10] Sim BK, Orlandi PA, Haynes JD, Klotz FW, Carter JM, Camus D, Zegans ME, Chulay JD: Primary structure of the 175K Plasmodium falciparum erythrocyte binding ntigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol 1990, 111:1877-84.
[11] Camus D, Hadley TJ: A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 1985, 30: 553-556.
[12] Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems E: Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 1995, 52:565-568.
[13] Mamillapalli A, Pattnaik P, Sharma M, Sharma SK, Tyagi PK, Joshi H, Chitnis CE: Sequence polymorphisms in the receptor-binding domain of Plasmodium falciparum EBA-175: Implications for malaria vaccine development. Mol Biol Parasitol 2006, 146: 120-123.
[14] Ntoumi F, Contamin H, Rogier C, Bonnefoy S, Trape JF: Mercereau-Puijalon, O. Age-dependent carriage of multiple Plasmodium falciparum merozoite surface antigen-2 alleles in asymptomatic malaria infections. Am J Trop Med Hyg 1995, 52:81-88.
[15] Robert F, Ntoumi F, Angel G, Candito D, Rogier C, Fandeur T, Sarthou JL, Mercereau-Puijalon O: Extensive genetic diversity of Plasmodium falciparum isolates collected from patients with severe malaria in Dakar, Senegal. Trans R Soc Trop Med Hyg 1996, 90:704-711.
[16] Cohen GH, Wilcox WC, Sodora DL, Long D, Levin JZ, Eisenberg RJ: Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol 1988, 62:1932–1940.
[17] Chitnis CE, Miller LH: Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med 1994, 180:497-506.
[18] Baum J, Thomas AW, Conway DJ: Evidence for diversifying selection on Erythrocyte-binding antigens of Plasmodium falciparum. Genetics 2003, 163:1327-1336.
[19] Liang H, Sim BK: Conservation of structure and function of the erythrocyte binding domain of Plasmodium falciparum EBA-175. Mol. Biochem. Parasitol. 1997, 84:241-245.
[20] Orlandi PA, Sim KL, Chulay JD, Haynes JD: Characterization of the 175–kilodalton erythrocyte binding antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 1990, 40:285–294.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors fully retain the ownership of their copyright and they may reuse their work in any way, including commercially. Once the paper is published, users are free to share license (i.e., copy and redistribute the contribution in any medium or format) and adapt (remix, transform, and build upon) non-commercial use of the material, under the Creative Commons CC BY with the following conditions: They must give appropriate credit to the contribution, provide a link to the license, and indicate if changes were made.