January 2020
May 15, 2020Proteomics, Digestive Diseases & Metabolism
June 8, 2020
Abstract
Custom-designed or tailoring of proteins for specific applications is a highly active and growing area of research. A major part of this research is focused on protein engineering especially protein interfaces and interactions by employing biomolecular engineering tools. This research focus is partly due to the significance of protein interactions that are known to communicate critical information from the environment into cells to mobilize functional responses relevant to health and disease. Protein engineering can lead to custom-designed protein structures and biomaterials with the desired affinity, specificity, mechanism, or other properties that are of practical biomedical interests. Here, we describe a brief overview of protein engineering and some of the recent breakthroughs in biomedical applications of designer proteins. We discuss recent applications in new vaccine designs and advanced therapeutics for battling the challenges of complex diseases.
1. Introduction
The need for specifically engineered and rationally designed proteins with different functionalities has started the fascinating field of protein engineering. This field has provided many breakthroughs so far with wide-scale application potentials in industrial, biotechnological, and pharmaceutical sectors. Ongoing studies have shown tremendous promise of protein based therapeutics, new vaccines, and novel protein scaffolds with greater safety, improved efficacy, reduced immunogenicity and superior drug delivery with innovative biomedical formulations [1, 2]. To this end, researchers have proposed strategies to engineer protein interactions to modulate function using molecular engineering and also tailoring protein networks that exhibit robust plasticity that enables reprogramming of receptor interactions [3].
It is believed that the design strategies of innovative protein interface and engineered bio-orthogonal protein networks will lead to the development of advanced biologics and next generation research tools and therapies. The ongoing research has therefore, focused on developing new tools for protein engineering to realize the vast potential of protein materials. One such technique is directed evolution that has emerged as a powerful tool to improve biological systems. Directed evolution works based on mutation and selection. In this process, the encoding of DNA is mutated, and subsequently the resulting variants are screened and selected for specific functionality. This approach presents a promising avenue to produce novel protein materials [4]. In addition to directed evolution, a combination of various protein engineering approaches has been considered to create protein variants with a wide range of desired properties [2].
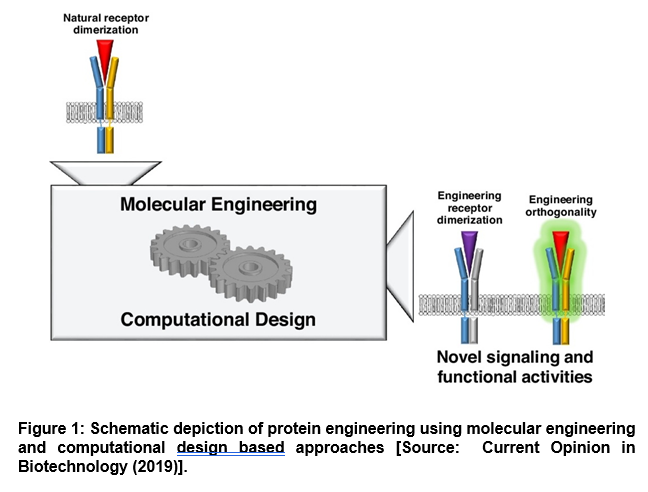
Further, computational methods have been shown to be of huge promise in protein engineering that can be leveraged for mechanism-driven interface design (Figure 1) [3]. To this end, researchers have integrated computational algorithms into the protein design process that has facilitated exciting new directions in immunotherapeutic development. For example, computational protein design can be employed to predict protein structures from amino acid sequences. This also includes engineering new proteins that enact desired functions. More importantly, it has been shown that computational methods can enable engineering of protein mechanisms, especially through design of neutralizing agents or allosteric modulators [3].
Here, we have described some of the important breakthroughs in protein engineering for new designs of vaccines and development of smart therapeutics.
2. Artificial Proteins to Design New Vaccines
Vaccines are known to trigger the immune system to produce antibodies. This protects humans against infection, and therefore, vaccines are considered the most effective interventions that prevent the spreading of infectious diseases. There has been an intense research focus on engineered protein designs particularly to obtain targeted neutralizing antibody (nAb) responses for the development of new vaccines. De novo proteins are those proteins that are designed from the ground up. They are considered promising candidates in such vaccine designs [5].
De novo proteins that mimic a viral epitope outside the context of the native protein are of special interests due to their potential applications as immunogens that can induce targeted virus neutralizing antibodies (nAbs) in-vivo. However, de novo proteins exhibit regular and continuous structural patterns that are limited to mimicking the simplest epitopes. Thus, it’s a challenge for the use of de novo proteins in epitope-focused immunogens that greatly limit their potential in the field of vaccine design [5].
Further, studies have shown that several major human pathogens only display a limited number of broadly neutralizing epitopes unlike respiratory syncytial virus (RSV) that are surrounded by strain-specific, non-neutralizing, or disease-enhancing epitopes [5]. This has presented a major research goal in engineering de novo proteins for vaccine development that seeks to trigger antibody responses with precisely defined epitope specificities. In addition, other specific requirements include constrained molecular features such as antibody lineage, complementarity-determining region length, or binding angle [5].
Researchers addressed these challenges and limitations by employing de novo design approaches to engineer epitope-focused immunogens that mimicked irregular and discontinuous RSV neutralization epitopes. They developed a strategy to design artificial proteins that very precisely instruct the body’s immune system which antibodies to produce [5].
In this strategy, researchers assembled protein topologies that were tailored to the functional motif. The aim was to enable the design of de novo proteins endowed with complex structural motifs. This approach was employed to develop an immunogen cocktail presenting three major antigenic sites of the respiratory syncytial virus (RSV) fusion protein (RSVF) in order to induce nAbs acting through precisely defined epitopes [5]. It was developed based on a novel computational design strategy, TopoBuilder, to build de novo proteins that presented complex structural motifs. TopoBuilder enabled to define and build protein topologies to stabilize functional motifs that were followed by in silico folding and sequence design using Rosetta (Figure 2) [5].
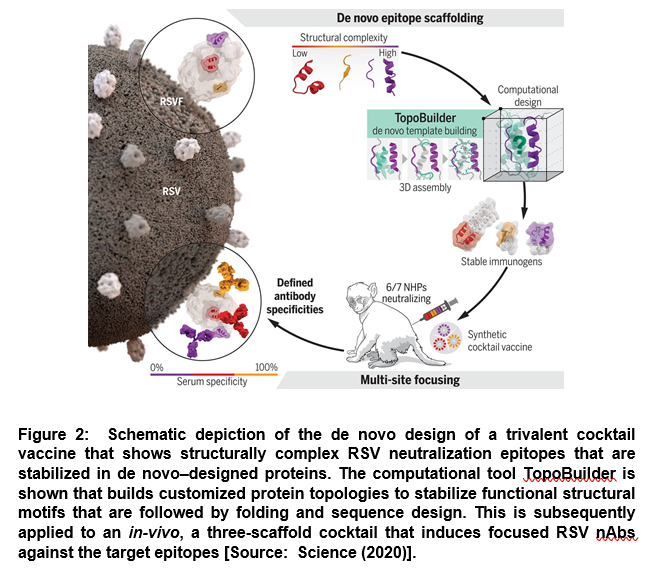
This study showed a new route to functionalizing de novo proteins that enables the assembly of customized protein topologies tailored to structural and functional requirements of the motif. This can be employed for epitope-centric vaccine design. Such a design strategy can be leveraged to gain control over induced antibody specificities in both naïve and primed antibody repertoires [5].
3. Protein Therapeutics for Smart, Stimulus-Responsive Drug Systems
In addition to new vaccine designs, one promising area of research in protein engineering is the development of smart, stimulus-responsive drug systems with improved clinical outcomes. This research has initiated a new field called protein therapeutics, which is expected to transform the metabolic drug landscape [6, 7]. Although, engineering of protein therapeutics is a relatively new field, important discoveries in protein engineering tools are enabling to gain improved control over both pharmacokinetics and pharmacodynamics that are essential to take protein therapeutics to the next level. For example, innovative drugs based on stimulus-responsive protein therapeutics have been designed to be metabolized under targeted conditions. Further, researchers are using protein engineering to develop tailored smart therapeutics with biochemical logic [6-8].
Researchers have studied antibody-drug conjugates (ADC) that are considered the simplest form of stimulus-responsive protein therapeutics. In this therapeutics, a monoclonal antibody is used to target the conjugated drug to specific locations, such as cancer cells. In this process, a sensitive linker connecting the antibody and drug allows selective release of the drug [6].
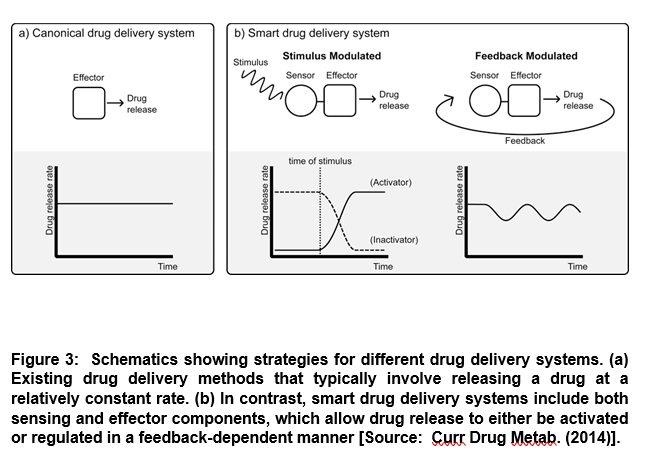
The emergence of smart response drug systems has provided an exciting frontier of drug development that leverages advanced protein engineering capabilities. Researchers have envisioned smart response drug systems that can utilize feedback mechanisms to intelligently modulate a therapeutic effect in response to biomarkers or other relevant stimuli [6]. It is known that a drug is released slowly at an approximately constant rate in many existing delayed drug release systems. On the other hand, smart drug delivery systems differ from the delayed drug release process. Smart systems require the presence of external stimuli to turn on the release of a drug in activation-modulated release systems. Such systems may also require to modulate the extent of a drug that is released in feedback-modulated release systems (Figure 3) [6].
4.Conclusion and Outlook
The fascinating research field of protein engineering involving medicine and in particular pharmacology has shown tremendous potential for an exciting transition from small molecule chemical therapy to biological therapy. This exciting developments span from protein therapeutics, gene therapy to cellular therapy, where protein engineering is a central and enabling technology for all these biological therapies to combat complex disease. Ongoing studies have suggested that for protein therapeutics, a robust control of half-life and immunogenicity needs to be achieved to accomplish clinical goals. It is anticipated that protein engineering will continue to impact these areas for next generation vaccine and therapeutics developments.
It is also anticipated that future therapeutics will be much broader by taking advantage of the full potential of protein engineering. In this regard, one area in engineering protein therapeutics with huge growth potential is expected to be stimulus-responsive for targeted activation and/or targeted neutralization, which will revolve around the modulation of protein function and stimulus-responsive function. This will further be impacted by the ongoing advances in gene therapy and other intracellular protein delivery methods that can improve various protein design constraints. Future research is expected to focus on better understanding of protein molecular recognition, allostery, and catalysis that will pave the way for further development of computational protein design methods.
References
[1] Malgosia M. Pakulska, Shane Miersch, Molly S. Shoichet, Designer protein delivery: From natural to engineered affinity-controlled release systems, Science, 351, Issue 6279, aac4750 (2016), DOI: 10.1126/science.aac4750.
[2] Brindha J., Balamurali M. M. and Kaushik Chanda, Evolutionary approaches in protein engineering towards biomaterial construction, RSC Adv., 9, 34720-34734 (2019),
DOI: https://doi.org/10.1039/C9RA06807D.
[3] Patrick J Krohl, Seth D Ludwig and Jamie B Spangler, Emerging technologies in protein interface engineering for biomedical applications, Current Opinion in Biotechnology 60:82–88 (2019), DOI: https://doi.org/10.1016/j.copbio.2019.01.017.
[4] Anton Kan and Neel S. Joshi, Towards the directed evolution of protein materials, MRS Commun., 9(2): 441–455 (2019), DOI: 10.1557/mrc.2019.28.
[5] Fabian Sesterhenn, Che Yang, Jaume Bonet, Johannes T. Cramer, Xiaolin Wen, and Bruno E. Correia, De novo protein design enables the precise induction of RSV-neutralizing antibodies Science, 368, Issue 6492, eaay5051 (2020), DOI: 10.1126/science.aay5051.
[6] Peter H. Tobin, David H. Richards, Randolph A. Callender,and Corey J. Wilson, Protein Engineering: A New Frontier for Biological Therapeutics, Curr Drug Metab., 15(7): 743–756 (2014), DOI: 10.2174/1389200216666141208151524.
[7] Steven M. Jay and Richard T. Lee, Protein Engineering for Cardiovascular Therapeutics, Circulation Research, 113, Issue 7, 13, 933-943 (2013), DOI: https://doi.org/10.1161/CIRCRESAHA.113.300215.
[8] Michaela Gebauer and Arne Skerra, Engineered Protein Scaffolds as Next-Generation Therapeutics, Annual Review of Pharmacology and Toxicology, 60:391–415 (2020), DOI: https://doi.org/10.1146/annurev-pharmtox-010818- 021118.