Designer Proteins for New Vaccines and Therapeutics
June 8, 2020Aging
June 8, 2020
Abstract
Lately, there has been a significant amount of research focus on circadian clock and its impact on human health. It has been shown that disruption of circadian rhythm could lead to cancer and metabolic syndrome. Researchers have identified in a number of murine studies the benefits of time-restricted feeding that can reset the disrupted clock rhythm, which can subsequently optimize the functioning of critical regulatory proteins of metabolism in mice. This has prompted a huge research interest in studying time-restricted feeding in humans for protective actions against cancer and metabolic syndrome and many other complex medical conditions. To this end, a hypothesis based on intermittent fasting for several consecutive days without calorie restriction in humans has been recently clinically tested that has shown an induced anti-carcinogenic proteome and the key regulatory proteins of glucose and lipid metabolism for a number of significant health benefits. Here, we present a brief overview on circadian rhythm and its disruption and also the effects of time-restricted feeding to reset the detrimental disruption. We describe recent clinical studies showing potential benefits of intermittent fasting as an adjunct therapy in a number complex medical conditions such as cancer, metabolic syndrome, and several cognitive and neuropsychiatric diseases.
- Introduction: Disruption in Circadian Rhythm and Health Consequences
Circadian rhythms are found in most living things. They correspond to physical, mental, and behavioral changes that follow a daily cycle. Studies have shown that circadian rhythm among mammals is controlled by an organized system comprising both central and peripheral oscillators that communicate via neural connections and hormonal cues [1]. Researchers have shown that the ability to adjust physiology and behavior in response to daily changes in the environment is critical for survival. Mammals achieve this ability via a master circadian clock in the suprachiasmatic nucleus (SCN) whose role is to coordinate rhythms in cells and tissue function. This occurs throughout the body according to predictable daily variations in individuals [2]. Further, the oscillators are known to be located within individual neurons in the SCN, where approximately 20,000 neurons are found within bilateral SCN. Also, there are peripheral clocks in addition to the master clock in the SCN that are found in a number of vital organs that include the liver, adipose tissue, kidneys, pancreas, and heart [1, 3].
Figure 1 shows schematically the core of the mammalian circadian clock that comprises a transcription–translation feedback loop involving the Period (Per1-3) and Cryptochrome genes (Cry1-2) [1]. It shows a heterodimer of transcription factors including clock and also BMAL1 drive the transcription of these target genes, which contain an E-box enhancer element (Figure 1). This results in PER and CRY proteins that subsequently inhibit the clock (BMAL1) complex. In addition to PER and CRY genes, it is shown that the clock (BMAL1) complex also drives the transcription of metabolic genes such as PPARα, Ror α/β (Ror), and Rev-Erb α (Rev) (Figure 1) [1].
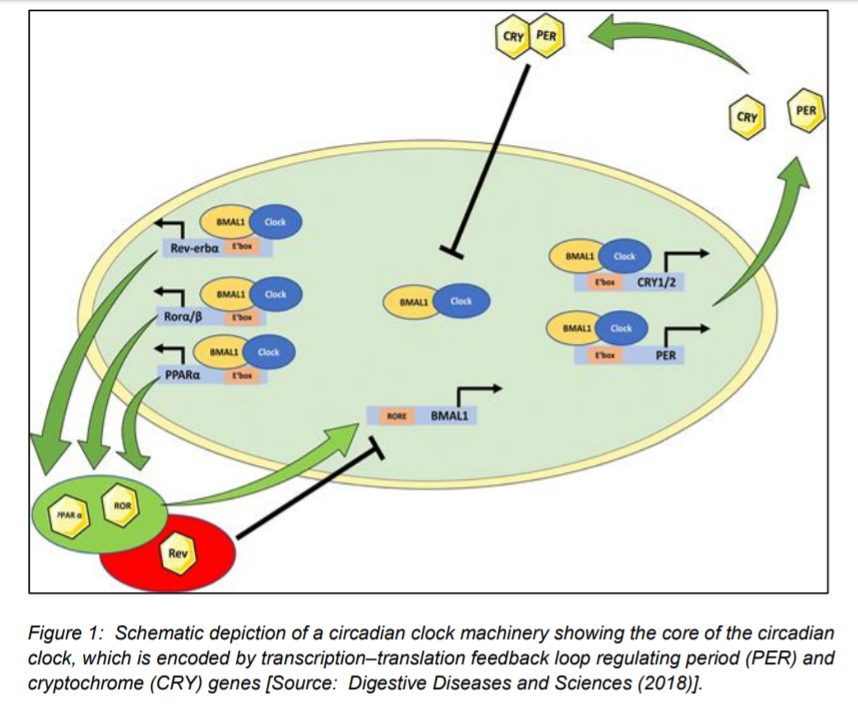
Researchers have studied the circadian rhythm that have shown that it helps the body anticipate environmental cues that is needed to optimize energy utilization for the diurnal cycles of rest–activity and feeding–fasting [3]. Further, murine studies have shown that disruption of circadian clock rhythmicity could lead to cancer and metabolic syndrome [4].
The reasons for the serious health consequences as a result of the disruption of circadian rhythm have been shown to be associated with alterations in glucose and lipid metabolism and immune system responses, and also carcinogenesis [3]. It has been suggested that adoption of a strategy based on resetting the disrupted rhythm of the circadian clock could help prevent metabolic syndrome, immune system dysfunction, and cancer [3].
Regarding primary mechanisms that govern the resetting of the circadian clock, one dominant mechanism has been proposed that functions through the master clock located in the SCN of the anterior hypothalamus. This works by dark-light cycles of the day. Subsequently, in this process, all peripheral clocks get synchronized by the master clock via neuronal and humoral signals. This process then results in resetting all peripheral clocks, including hepatic clock during ad libitum food consumption. The other mechanism to reset the circadian clock is considered a secondary mechanism, which is based on the response to mealtime during rhythmic, consecutive and time-restricted feeding-fasting cycles [3, 5].
- Time-Restricted Feeding to Reset Disrupted Clock in Humans
Studies have shown the potential of time-restricted feeding to reset the disrupted clock rhythm that could protect against cancer and metabolic syndrome. In this regard, several murine animal model studies have been carried out that have shown the positive effects of time-restricted access or no access to food during night time/dark phase on resetting the phase of the hepatic clock. This has been shown to optimize the amplitude of hepatic clock oscillations. This subsequently results in the upregulation of mRNA and various protein synthetic pathways especially including enzymes that can play a crucial role in carbohydrate and lipid metabolism [3]. However, the murine animal studies employing mice could not address the potentials of time-restricted feeding occurring during the daytime. This is due to the reason that mice are nocturnal feeders, and they consume food mostly at night. On the other hand, in the case of humans, meal intake and most activity usually occur during the daytime [6].
It is of huge clinical significance to study the potentials of time-restricted feeding in humans that allows to reproduce similar optimization in key metabolic regulatory proteins in humans. However, it requires that fasting should occur during the daytime activity for several consecutive days [3]. Further, in such a study, it is of major importance to preserve daytime activity and also timing of the major food consumption at transition zones of the day. In addition, a predawn breakfast and dinner at sunset may be also considered in the time-restricted feeding study due to the significance of caloric content and composition of the food in the prevention of metabolic syndrome and its complications and cancer [3, 6].
We present in the following section recent clinical results on intermittent fasting from dawn to sunset that have shown several health benefits including prevention of cancer, metabolic syndrome and Alzheimer’s disease and several neuropsychiatric diseases.
- Human Study of Serum Proteomics: Important Clinical Implications and Health Benefits
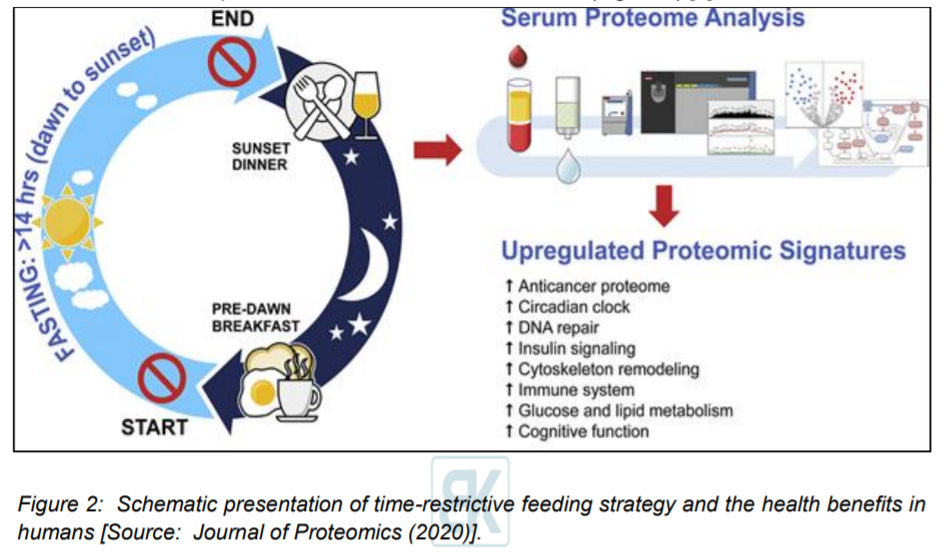
Researchers conducted the first human study of serum proteomics that included a 30 day dawn-to-sunset intermittent fasting (Figure 2). This study also simultaneously included assessment of clinical metabolic parameters, multiple serum biomarkers, and fecal microbiota in 14 healthy subjects [3].
This study was based on the hypothesis that intermittent fasting for several consecutive days without calorie restriction in humans would induce an anti-carcinogenic proteome and the key regulatory proteins of glucose and lipid metabolism [3]. It involved fourteen healthy subjects that were fasted from dawn to sunset for over 14 h daily and the fasting duration was continued for 30 consecutive days [3]. Researchers collected serum samples before 30-day intermittent fasting, which occurred at the end of 4th week during 30-day intermittent fasting, and one week after 30-day intermittent fasting [3]. Subsequently, they performed an untargeted serum proteomic profiling using ultra high-performance liquid chromatography/tandem mass spectrometry. Researchers demonstrated that 30-day intermittent fasting was associated with an anticancer serum proteomic signature. Also, upregulated key regulatory proteins of glucose and lipid metabolism was shown along with circadian clock, DNA repair, cytoskeleton remodeling, immune system, and cognitive function. This resulted in a serum proteome protective against cancer, metabolic syndrome, inflammation, Alzheimer’s disease, and several neuropsychiatric disorders [3].
These findings suggested the major clinical significance and positive impact on health as a result of fasting from dawn to sunset for 30 consecutive days. Especially, it is noteworthy that these findings were obtained in the absence of any calorie restriction and significant weight loss [3]. Researchers envisioned that such a time-restrictive feeding strategy could be employed in humans as adjunct therapeutic measures for the prevention of cancer, obesity, diabetes, metabolic syndrome, and several cognitive and neuropsychiatric diseases (Figure 2) [3].
- Conclusion and Outlook
Research has revealed that the alterations in glucose and lipid metabolism and immune system responses, and carcinogenesis are associated with the disruption of circadian rhythm. Important clinical studies based on intermittent fasting from dawn to sunset have shown novel therapeutic pathways in the prevention of metabolic syndrome, immune system dysfunction, and cancer by resetting the disrupted rhythm of the circadian clock. This innovative therapeutic approach offers promise in the prevention of cancer as well as in several metabolic, inflammatory and immune diseases, Alzheimer’s disease and also neuropsychiatric disorders. Recent research has shown that such a strategy based on time-restricted feeding in humans can potentially trigger the important proteome that is protective against carcinogenesis, obesity, diabetes, metabolic syndrome, inflammation, cognitive dysfunction, and mental health.
The existing research data shed light on the functionality of the circadian clock system and metabolic homeostasis. These were shown to be tightly linked and characterized by an extensive crosstalk. However, existing data do not give any clue on the mechanisms of this interaction. These mechanisms are still not understood clearly due to the fact that both aspects of physiology are linked through a complex array of functions. Further research into this fundamentally important area could be undertaken. Future research could also focus on targeting the circadian clock-controlled genes or nuclear receptors, which affect metabolic pathways. This would be useful to pave the way for new therapies in metabolic syndromes and related diseases.
References
[1] Akshay Shetty, Jennifer W. Hsu, Paul P. Manka, Wing‑Kin Syn, Role of the Circadian Clock in the Metabolic Syndrome and Nonalcoholic Fatty Liver Disease, Digestive Diseases and Sciences 63:3187–3206 (2018), DOI: https://doi.org/10.1007/s10620-018-5242-x.
[2] Paul, S., Hanna, L., Harding, C. et al. Output from VIP cells of the mammalian central clock regulates daily physiological rhythms, Nat Commun 11, 1453 (2020), DOI: https://doi.org/10.1038/s41467-020-15277-x.
[3] Ayse L. Mindikoglu, Mustafa M. Abdulsada, Antrix Jain Jong Min Choi Prasun K. Jalal Sridevi Devaraj Melissa P.Mezzari Joseph F.Petrosino, Antone R. Opekun, Sung YunJung, Intermittent fasting from dawn to sunset for 30 consecutive days is associated with anticancer proteomic signature and upregulates key regulatory proteins of glucose and lipid metabolism, circadian clock, DNA repair, cytoskeleton remodeling, immune system and cognitive function in healthy subjects, Journal of Proteomics, 217, 103645 (2020), DOI: https://doi.org/10.1016/j.jprot.2020.103645.
[4] Michael W. Greene, Circadian rhythms and tumor growth, Cancer Letters, 318, 2, 115-123 (2012), DOI: https://doi.org/10.1016/j.canlet.2012.01.001.
[5] J.A. Evans, Collective timekeeping among cells of the master circadian clock, J. Endocrinol., 230 (1), R27-R49 (2016), DOI: doi:10.1530/JOE-16-0054.
[6] A.L. Mindikoglu, et al., Impact of time-restricted feeding and dawn-to-sunset fasting on circadian rhythm, obesity, metabolic syndrome, and nonalcoholic fatty liver disease, Gastroenterol. Res. Pract., 3932491 (2017), DOI: https://doi.org/10.1155/2017/3932491.