Ischemic stroke is a serious medical condition and widely considered one of the most common causes of death and disability in the world today. There have been notable research advances in stroke so far and studies have shown that stroke’s complex pathophysiology process involves the oxidative stress and inflammatory reaction. However, despite the progress in stroke research, currently there are no established biochemical factors available that can be employed in the early diagnostics and intervention in stroke. Mostly, stroke diagnosis is based on neuroimaging, which is not a rapid tool to diagnose stroke. This decreases the survivability rate. Further, conventional therapeutic approaches for ischemic stroke management are based on restoring blood flow to the affected brain area and these therapies are effective only during a limited time window. Hence, this procedure results in benefiting only a small percentage of patients. In view of these limitations, the ongoing research has focused on seeking alternative treatment methods that can reduce stroke brain damage and improve patients’ outcome. To this end, research goals are targeted towards gaining insights into the inflammatory response triggered by cerebral ischemia that is supposed to play an important role in the progression of stroke, and the subsequent study of inflammatory molecules in the acute phase of stroke. In this mini-review, we describe the inflammatory processes occurring during ischemic stroke along with the potential for pro-inflammatory cytokines to become stroke biomarkers as well as interesting neuroprotective therapeutic targets that could be blocked or stimulated to modulate inflammation after stroke. Finally, we present a perspective briefly discussing some viewpoints on future studies in the ongoing field of stroke research.
Keywords: Cytokines; Pro-inflammation; Stroke; Biomarkers; Therapeutics
*E-mail: meghaagra@gmail.com; megha@biotechkiosk.com
To cite this article: Agrawal M, Pro-Inflammatory Cytokines as Potential Early Biomarkers and Therapeutic Targets to Combat Ischemic Stroke, Biotechnol. kiosk, Vol 2, Issue 11, PP: 5-19 (2020); DOI: https://doi.org/10.37756/bk.20.2.11.1
Introduction
Ischemic stroke represents a serious medical condition that is known to cause disability in elderly people (over 65 years of age) and considered the third most common cause of death in the world. According to an estimate by the World Health Organization, one in six people globally can be vulnerable to suffer from stroke in their lifetime. Ischemic stroke is known to be the most frequent kind of stroke that affects approximately 85–90% of patients. It can be caused by any of the medical conditions that include cardiogenic embolism, cerebral microcirculatory impairment or cerebral microangiopathy, atherosclerosis of extra- and intracranial arteries, and also blood clotting disorders that have been suggested as the cause of ischemic stroke [1-4].
The pathophysiology of stroke has been a subject of immense research interest. Studies conducted on stroke pathophysiology have suggested it to be a complex process and the key mechanisms involved in neuronal damage are suggested to be constituted by oxidative stress and inflammatory reaction [5]. It is understood that brain and immune cells produce reactive oxygen species (ROS) during ischemia and asphyxia. Such reactive species stimulate endothelial cells and cause oxidative stress. Consequently, it has been observed that the activity of ROS during ischemic stroke can cause primary vascular damage. It can also trigger the development of the inflammatory response that is connected with the acute immune response. In this process, glial cells (microglia, astrocytes) that are activated in ischemia together with blood cells (leukocytes) and endothelial cells have been shown to synthesize a number of biochemical mediators and markers of inflammation including cytokines, chemokines and pro-inflammatory enzymes. Further, some genetic factors have been found to play significant roles in the development of inflammation in stroke that results in inflammatory reactions. In this regard, many genes have been demonstrated that can contribute to both the etiology of stroke and the size of the ischemic area, which affects the patient’s late outcome. All these suggest that chronic activation of inflammation can be a factor that can significantly influence the advancement and impact of stroke risk factors [5-7].
While, there are notable advances made in a broad range of molecular diagnostic methods, biochemical factors that exhibit proven benefits for the rapid and early diagnostics, intervention and treatment of stroke have not yet been clearly established. To bridge the gap, studies related to the diagnostic and prognostic value of pro-inflammatory factors of neuronal, glial and neuroendocrine origin along with inflammatory markers in ischemic stroke are of huge current research interests [6, 7]. A special research focus is on cytokines, as regulatory proteins that are produced by different types of cells. These cytokines are recognized and bound by specific receptors that are located on cell membranes. A number of pro-inflammatory cytokines are known that include chemokines, interleukins, interferons, as well as cell differentiation and growth factors. Studies have suggested that they can transduce signal to the cell nucleus by binding to the receptor in which expression of genes encoding acute phase proteins (APPs) occurs. In addition, they can be triggered by the transcription factor (NF-κB). Further, it has been found that the inflammatory response in the brain changes over time. These observations imply that the knowledge concerning if and when the level of cytokine increases or decreases in the course of the acute phase of cerebral stroke could be immensely helpful that could pave the way to determine a possible role of pro-inflammatory cytokines as early biomarkers and also the target of a potential therapeutic intervention in the acute phase of cerebral stroke [8-11].
Here, we present an overview of the role of pro-inflammatory cytokines as early inflammatory factors for possible stroke biomarkers and novel therapeutic targets. Cytokines that could potentially act as different phases of inflammatory markers and influence the final prognosis could very well provide an alternative treatment method for stroke.
Stroke Biomarkers and Therapeutic Targets: Technology Challenges and New Innovations in Stroke Management
Biomarkers have huge pharmacological and clinical significance as they are important indicators of normal biological processes, pathogenic processes, or pharmacologic responses to therapeutic interventions. They can be objectively measured and evaluated as characteristics of important biomedical processes. Clinical biomarkers are employed for practical applications of diagnostics. These biomarkers can be detectable molecules from different biological fluids such as blood, urine, or saliva. To be clinical-grade biomarkers, it is extremely important that they have high specificity and sensitivity for the indicated process together with accuracy in their measurements and reproducibility, and easily interpretable by clinicians [10, 12, 13].
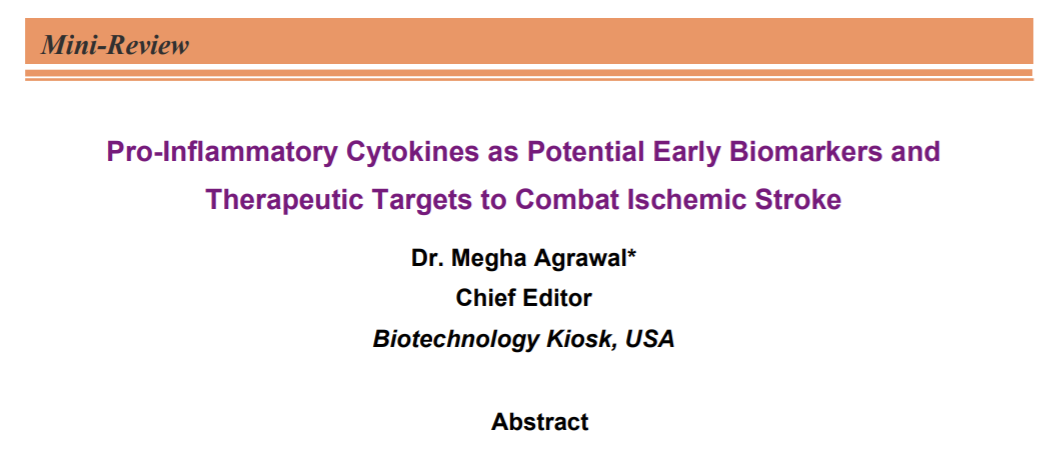
With respect to stroke biomarkers, they are representative of the brain injury processes triggered by stroke. For clinical applications, stroke biomarkers are considered in different phases that include both the diagnosis and the prognosis of stroke. Further, in the case of ischemia, the first few days after the onset of stroke require most important therapeutic decisions that need rapid biomarkers [13]. However, in practical applications, there is a possibility that stroke biomarkers may change dramatically in a short window of time especially during the hyper acute and acute phases compared with other phases. This poses a challenge for identifying optimal treatment windows for clinical biomarkers in each phase of stroke and stroke subtype (Figure 1) [13].
While, there has been some research progress in this direction, no biomarkers for stroke are currently available to use in clinical practice [12]. This is a big technology gap that currently exists. As regards to new ideas and looking ahead, researchers have shown that blood brain barriers ‘BBB’ disruption favors the release of brain antigens into the peripheral circulation. This event makes these molecules promising stroke-associated biomarkers. These biomarkers could reflect in the circulatory system, where pathological processes take place in brain after the ischemic event. Further, another thought is that both local and peripheral inflammatory response could produce systemic indicators that might serve as stroke biomarkers [10].
Recent research innovations in high-throughput technologies has paved the way to advance our understanding of the pathophysiology of this complex disease. It has allowed generating a significant amount of data and information at different molecular levels. It is believed that the integration of these multi-omics data can be leveraged to study thousands of proteins (proteomics), genes (genomics), RNAs (transcriptomics) and metabolites (metabolomics). Concurrent studies of these multi-omics data are expected to lead to revealing interaction networks between the molecular levels. It is believed that the strategy based on integrated analysis of multi-omics data using proteomics, metabolomics, transcriptomics and genomics would pave the way to useful insight into stroke pathogenesis, identification of therapeutic targets and biomarker discovery [12].
While different mechanisms are thought to be involved in the pathophysiology of stroke due to its complexity, there is an increasing evidence that suggests an important role of inflammation in the progression of stroke. This leads to a rapid growth in the research in therapeutic targeting of post ischemic inflammation in acute stroke as a potential neuroprotective strategy [11]. Especially, integrated analysis that combines proteomics, genomics, transcriptomics and metabolomics is considered to extend to study pro-inflammatory cytokines that have been found altered after ischemic stroke and have shown a potential role in the detection and modulation of inflammation. This could make them attractive candidates for both potential biomarkers and novel therapeutic targets for stroke management [10, 11].
The Occurrence and Role of Inflammatory Condition in Ischemic Stroke
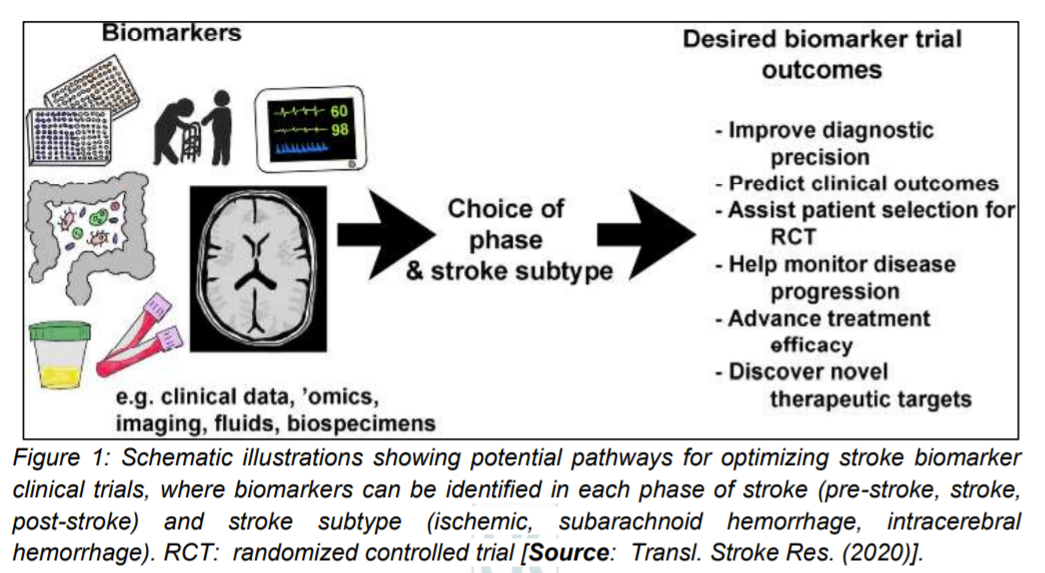
The biochemical processes of pathophysiology of ischemic stroke of the brain correspond to a complex cycle that include interconnected molecular and cellular mechanisms (Figure 2) [11]. Previous studies have suggested that there is a vital role of an inflammatory condition at all stages of the ischemic cascade. This role involves right from the starting from the cessation of flow to the late regenerative processes that are associated with the repair of ischemic tissues [10, 11]. The main mechanism of cell death in cerebral infarction is supposed to be the energy deficit caused by the loss of neurons ability to synthesize ATP (adenosine triphosphate). Further, it has been observed that while there is an increased extracellular concentration of K+ ions and the uncontrolled influx of Na+, Ca2+, Cl− ions, the activity of Na+/K+ – ATPase decreases in this process. Subsequently, loss of membrane potential along with gradual depolarization of the cell membrane occurs. This results in an increased flow of sodium that allows for transport of water to cells by an osmotic process, which leads to the development of cytotoxic edema. Consequently, loss of membrane integrity and cell death occurs as a result of the accumulation of Na+ and Ca2+ ions leading to organelle degeneration. Catabolic enzymes are activated by the influx of Ca2+ ions. This happens by the production of arachidonic acid and increasing the production of ROS mainly in neurons (Figure 2) [11, 14, 15].
An increased production of ROS triggers a cascade of events. First, it enables the passage through the membrane of ions and low-molecular-weight substances that cause necrosis. Secondly, depending on the degree of neurons damage, it leads to a programmed death of the nerve cell. Further, studies have shown that the excitotoxicity and an increased ROS results in activating microglia and astrocytes, which secrete cytokines, chemokine and matrix metalloproteinases (MMP) (Figure 2) [10, 11]. Consequently, these inflammatory mediators have been shown to induce the expression of cell adhesion molecules on the endothelial surface (P-selectin, E-selectin, endothelial-leukocyte adhesion molecule (ELAM-1) and intercellular cell adhesion molecules-1 (ICAM-1)), which have been attributed as enabler of the neutrophils to infiltrate ischemic areas of the brain [10].
Furthermore, this cascade leads to the release of hazard signals by necrotic neurons that in turn activates the immune system and secrete molecular patterns (DAMP). DAMP then induces the Toll-like receptors (TLRs) on microglia that stimulates the NF-κB to synthesize most of the pro-inflammatory cytokines (Figure 2) [11]. Additionally, NLRP3 inflammasome activates caspase-1 that subsequently results in maturation and secretion of IL-1β and IL-18 cytokine. Pro-inflammatory cytokines (IL-1β, interleukin 6 (IL-6), IL-18 and tumor necrosis factor (TNF-α)) and chemokines are produced by M1 microglia, while M2 microglia produce anti-inflammatory cytokines (interleukin 10 (IL-10), interleukin 4 (IL-4) and transforming growth factor-beta (TGF-β)). These anti-inflammatory cytokines are released a few days after acute brain damage that lead to inhibition of inflammation [16, 17].
As Figure 2 schematically illustrates, MMP which is secreted by microglia, astrocytes and neurons mediates in the destruction of the base plate. This results in increasing BBB permeability that facilitate the entrance of additional peripheral immune cells into the brain area affected by stroke. This phenomenon is attributed to two factors. It is suggested that acute ischemia of the brain not only causes a local inflammatory reaction, but it also triggers a systemic immune response [11]. Studies have shown that both congenital and adaptive immunity are involved during the stroke. However, they do not affect the acute phase of damage. But, according to latest theories, it is considered that modulation of adaptive immunity can have a significant protective effect on the ischemic brain. This creates bright prospects for new stroke therapies. However, it is also speculated that such immunomodulation could produce some side effects as well. Therefore, it is thought that a better understanding of the interaction between the immune system and the ischemic brain is essential that can help exploit the full therapeutic potential of stroke immunology [10, 14-17].
Immune System in the Production of Pro-Inflammatory Cytokines
A significant amount of research is currently underway that involves clinical and experimental studies to gain insights into the complex role of the immune system in pathophysiological changes that occur after acute ischemic stroke of the brain. These studies are targeted to better understand molecular signals that are generated by cerebral ischemia. Consequently, the activated components of the congenital immune system lead to amplification of inflammatory cascades and tissue damage, while these processes simultaneously stimulate a potentially harmful and cytoprotective immune adaptive response [11, 18].
There are a number of cells that are involved in the production of cytokines and are representatives of the congenital and adaptive immune response. These include microglia that are a type of resident marrow cells of central nervous system (CNS). Microglia’s main roles correspond to removing debris, repairing damaged cells and also acting as CNS homeostasis regulator. They produce pro-inflammatory mediators such as TNF‐α, IL‐1β, IL-6 and ROS, NO after a stroke (Figure 2). Notably, studies have suggested their important contribution to the inhibition of inflammation and tissue repair [17, 19, 20]. Also, it has been studied that perivascular macrophages occur between the vascular primary membrane and the brain surface, where M1 macrophages are known to produce pro-inflammatory cytokines (IL-1β, interleukin 12 (IL-12), interleukin 23 (IL-23) and TNF-α), chemokine, ROS and NO (Figure 2), which promote the immune response of helper T cells type 1 (Th1) [17]. Further, mastocytes or mast cells (MC) that are found in meninges and cerebral vessels have been found to store vasoactive substances (histamine), anticoagulants (heparin), protease (MMP2, MMP9) and cytokines (TNF‐α). It has been reported that they are capable of phagocytosis, antigen presentation that can modulate the adaptive immune response [21]. Two different populations of tissue macrophages are classical and pro-inflammatory monocytes that participate in the production of TNF‐α [17]. It has been observed that after ischemia of the brain, quick recruitment of inflammatory monocytes happens to the site of damage where they activate macrophages [21].
Researchers have shown a combination of congenital and adaptive immunity dendritic cells (DC) produce antigen-presenting cells (APC) that generate antigen, containing a MHC class II antigen complex. The presentation of T-cells antigen CD4+ enables a process of clonal expansion in lymphatic organs promoted by autocrine interleukin 2 (IL-2) production [17]. Further, it has been shown that T cells cause ischemic damage after entering the brain parenchyma. This happens when T cells release pro-inflammatory agents and cytokines such as ROS, interferon γ (IFN-γ), TNF-α, IL‐1β, interleukin 17 (IL‐17) and interleukin 21 (IL‐21). Auxiliary T cells (Th) are known to be responsible for coordinating and modulating immune responses and depending on molecular signals, different subpopulations of Th-cell effectors may develop that can produce cytokines. Type 1 (Th1) cells produce IFN-γ and TNF and their development is supported by IL-12. These cells have been observed to produce cytotoxic cytokine IL-17 that contribute to acute ischemic brain damage [17, 22-24].
Which Inflammatory Cytokines can be targeted to Develop Stroke Biomarkers and Therapeutics for Clinical Trials?
A large body of research evidence suggests that ischemic stroke condition could be attributed to the induction of cytokines and chemokines that may contribute to the pathogenesis of adverse remodeling, and systolic and diastolic dysfunction. It has been discussed in detail in many research reports that inflammatory cytokines modulate phenotype and function of all myocardial cells, suppressing contractile function in cardiomyocytes, inducing inflammatory activation in macrophages, stimulating microvascular inflammation and dysfunction, and promoting a matrix-degrading phenotype in fibroblasts [25]. Therefore, the research focus has been on the important pro-inflammatory cytokines, interleukin-1 (IL-1), which is produced in the CNS by microglia, astrocytes, endothelium and neurons. This cytokine has two different forms that include one intracellular (IL-1α) and one secreted (IL-1β). It is believed that both forms act through the IL-1 type I receptor (IL-1RI), which is expressed by immune and endothelial cells [10]. Further, the pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 have been extensively implicated in the pathogenesis involving ischemic stroke and it is thought that they could potentially be very effective stroke biomarkers as well as novel therapeutic targets [10, 25].
Recent research has suggested that TNF-α-mediated adverse remodeling and heart failure progression may involve effects on cardiomyocytes, macrophages, and the extracellular matrix [25]. The inhibition of TNF-α seems to be a promising tool for stroke treatment. In a clinical trial with targeting the IL-1 system in human heart failure patients, treatment of patients with prior myocardial infarction and evidence of active inflammation with the anti-IL1β monoclonal antibody demonstrated favorable effects that showed reduced risk of the composite endpoint (non-fatal myocardial infarction, non-fatal stroke or death) by 15% in comparison with standard treatment. The study supported the role of IL-1β in the pathogenesis of atherothrombotic disease and also generated observations that may be important in understanding and treating heart failure [26-28]. These data suggest high promise of IL-1 and TNF-α and to corroborate the findings, it could be worth to perform a clinical trial in ischemic stroke patients to evaluate the biomarkers and therapeutic potential of inhibiting IL-1 and TNF-α [10, 25].
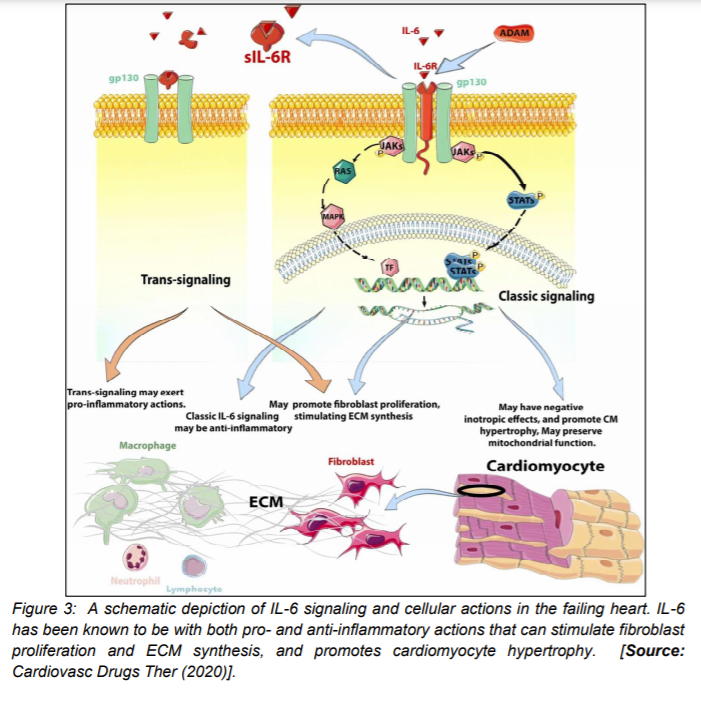
Another inflammatory cytokine, IL-6 has been shown to exhibit important modulatory effects on several different cell types in heart failure. Both pro- and anti-inflammatory effects of IL-6 are observed. Trans-signaling has been suggested to exert pro-inflammatory actions, whereas canonical IL-6 signaling may be anti-inflammatory [25]. Recent studies have shown that IL-6 exerts negative inotropic effects in cardiomyocytes and promotes a hypertrophic response through the gp130/STAT3 pathway. However, it has been observed that it may also exert protective actions, mediated through preservation of mitochondrial function (Figure 3) [25]. For example, IL-6 promotes proliferation and stimulates ECM synthesis in fibroblasts. It is believed that the combined effects of IL-6 on cardiomyocytes and fibroblasts could play an important role in the pathogenesis of HFpEF. Additionally, it has been reported that IL-6 has potent modulatory effects on macrophages and lymphocytes. The in-vivo role of IL-6 in inflammation has been found to be dependent on the relative contribution of macrophage and lymphocyte subsets [29-34].
Studies related to targeting IL-6 in human heart failure have suggested extensive evidence of a crucial role of IL-6 in the post-inflammatory hepatic acute phase response, which is implicated in autoimmunity. Based on this data, IL-6 has been considered by the researchers as an attractive therapeutic target in many conditions associated with inflammation [25]. In a clinical trial, the IL-6 receptor neutralizing antibody tocilizumab was approved as effective therapy for patients with moderate to severe rheumatoid arthritis and temporal arteritis, and for the treatment of the cytokine release syndrome associated with CAR-T cell therapies. Therefore, experimental evidence demonstrating involvement of IL-6 signaling in the pathogenesis of heart failure has suggested that treatment with tocilizumab may also be effective in heart failure patients [25, 34]. Thus, all data suggest that IL-6 could potentially be a good biomarker for stroke diagnosis and prognosis. However, further studies need to be done to better evaluate its therapeutic effect [10].
With respect to other anti-inflammatory cytokines, such as IL-4 and IL-10, all experimental studies seem to indicate that the administration of IL-10 has a neuroprotective effect, but its application as a new stroke therapy needs to be further studied before its translation to the clinical setting [10].
Conclusion and Future Perspective
Recent research advances in stroke have clearly implicated inflammation in the pathophysiology of stroke. Research evidence points out to the dual role of inflammation during ischemic stroke, which displays both beneficial and also adverse effects according to the phase of the stroke. Anti-inflammatory strategies have been suggested as promising tools for regulating the inflammatory processes that take place during the acute phase of stroke. To this end, several therapeutic strategies have been shown to be effective in both experimental models of ischemia and clinical trials. However, the realization of translation from bench-to-bedside is yet to happen in stroke research. In this context, the current focus is to gain a better understanding of the inflammatory mechanisms involved during ischemic stroke that would make the inflammatory cytokines as not only possible biomarkers but potential therapeutic targets. The knowledge of modulation of the possible therapeutic targets could be leveraged in the fight against the disease and its complications.
As regards the future studies, one area could be developing sensitive and rapid blood tests to diagnose and aid stroke prognostication, which is currently lacking and a major cause of concern world-wide. A number of advantages could result from the elaboration of robust biomarkers for stroke. To this end, future research might focus on their discovery. Effective stroke biomarkers would not only provide potential methods for stroke diagnosis and prognosis, but they would also contribute to the understanding of the pathophysiology of this devastating disease.
As the previous studies have shown, the inflammatory response to stroke is extremely complex, with multiphasic pro-inflammatory responses. So, future studies may focus on the determination of cytokine levels in the acute phase of stroke that can contribute to a thorough understanding of their role in the immune response observed during brain ischemia. Such an advancement can also be leveraged for the identification of interactions between the immune system and brain cells during post-stroke recovery and regeneration. Therefore, they would serve as prognostic factor in acute cerebral ischemia. With respect to inflammatory cytokines to be developed as biomarkers, IL-1 and IL-6 potentially appear as good prognostic factor in stroke. However, further research is necessary to assess the therapeutic implications of these cytokines. Further, research could focus on facilitating experimental models based on inhibition of TNF-α, CCL-2, CCL-3 and CCL-5 that could be a promising tool in the treatment of acute stroke. Moreover, as the current research data indicate, administration of the recombinant human IL-1 receptor antagonist may have important implications in reducing the inflammatory response. However, more clinical trials in patients with ischemic stroke are necessary in order to evaluate the therapeutic the cytokines potential inhibition.
For successful practical applications of inflammatory cytokines based stroke management, successful implementation of cytokine targeting approaches in heart failure patients is essential. This will require dissection of specific pro-inflammatory pathways with a critical role in dysfunction and progression of adverse remodeling. This also includes identification of patient subpopulations with dysregulated or overactive inflammatory responses that may derive maximal benefit from targeted cytokine or chemokine inhibition in effective stroke treatment.
References
[1] Mira Katan, Andreas Luft Global Burden of Stroke, Semin Neurol, 2018 Apr;38(2):208-211, doi: 10.1055/s-0038-1649503.
[2] Wein T, Lindsay MP, Côté R, et al. Canadian Stroke Best Practice Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke. 2018;13:420–443. doi:10.1177/1747493017743062.
[3] Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American heart association/American stroke association. Stroke. 2014;45:315–353. doi:10.1161/01.str.0000437068.30550.cf
[4] Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics —2018 update: a report from the American Heart Association. Circ Res. 2018;137:67–492.
[5] Lakhan, S.E., Kirchgessner, A. & Hofer, M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7, 97 (2009). DOI: https://doi.org/10.1186/1479-5876-7-97
[6] Powers WJ, Rabinstein AA, Ackerson TI, et al. American Heart Association Stroke Council: 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:46–110. doi:10.1161/STR.0000000000000158
[7] Simats A, García-berrocoso T, Montaner J. Neuroinflammatory biomarkers: from stroke diagnosis and prognosis to therapy. BBA Mol Basis Dis. 2016;1862:411–424. doi:10.1016/j.bbadis.2015.10.025
[8] Rose JS, Scheller J, Elson G, et al. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukocyte Biol. 2006;80:227–236. doi:10.1189/jlb.1105674
[9] Abayev M, Rodrigues JP, Srivastava G, et al. The solution structure of monomeric CCL5 in complex with a doubly sulfated N-terminal segment of CCR5. FEBS J. 2018;285:1988–2003. doi:10.1111/febs.14460
[10] Laura Ramiro, Alba Simats, Teresa García-Berrocoso, and Joan Montaner, Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management, Ther Adv Neurol Disord. 2018; 11: 1756286418789340, DOI: 10.1177/1756286418789340.
[11] Pawluk H, Woźniak A, Grześk G, Kołodziejska R, Kozakiewicz M, Kopkowska E, Grzechowiak E, Kozera G. The Role of Selected Pro-Inflammatory Cytokines in Pathogenesis of Ischemic Stroke. Clin Interv Aging. 2020;15:469-484, DOI: https://doi.org/10.2147/CIA.S233909.
[12] Montaner, J., Ramiro, L., Simats, A. et al. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat Rev Neurol 16, 247–264 (2020). https://doi.org/10.1038/s41582-020-0350-6.
[13] Simpkins, A.N., Janowski, M., Oz, H.S. et al. Biomarker Application for Precision Medicine in Stroke. Transl. Stroke Res. 11, 615–627 (2020). https://doi.org/10.1007/s12975-019-00762-3
[14] Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi:10.1016/j.neuron.2010.07.002
[15] Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:232–240. doi:10.1038/sj.bjp.0706400.
[16] Bustamante A, Simats A, Vilar-bergua A, et al. Blood/brain biomarkers of inflammation after stroke and their association with outcome: from C-reactive protein to damage-associated molecular patterns. Neurotherapeutics. 2016;13:671–684. doi:10.1007/s13311-016-0470-2.
[17] Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi:10.1038/nm.2399.
[18] Chamorro Á, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi:10.1038/nrneurol.2012.98.
[19] Amantea D, Micieli G, Tassorelli C, et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci. 2015;9:1–19. doi:10.3389/fnins.2015.00147.
[20] Rayasam A, Hsu M, Kijak JA, et al. Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology. 2018;154(3):363–376. doi:10.1111/imm.2018.154.issue-3.
[21] Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi:10.1196/nyas.2008.1143.issue-1.
[22] Felger JC, Abe T, Kaunzner UW, et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24(5):724–737. doi:10.1016/j.bbi.2009.11.002.
[23] Wan YY. Multi-tasking of helper T cells. Immunology. 2010;130:166–171. doi:10.1111/imm.2010.130.issue-2.
[24] Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gamma delta T cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15(8):946–950. doi:10.1038/nm.1999.
[25] Hanna, A., Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc Drugs Ther 34, 849–863 (2020). https://doi.org/10.1007/s10557-020-07071-0.
[26] Harouki N, Nicol L, Remy-Jouet I, Henry JP, Dumesnil A, Lejeune A, et al. The IL-1beta antibody gevokizumab limits car[1]diac remodeling and coronary dysfunction in rats with heart fail[1]ure. JACC Basic Transl Sci. 2017;2:418–30. https://doi.org/10. 1016/j.jacbts.2017.06.005.
[27] Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. https://doi.org/10.1056/NEJMoa1707914.
[28] Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–99. https://doi.org/10.1161/ CIRCULATIONAHA.118.038010.
[29] Melendez GC, McLarty JL, Levick SP, et al. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–31. https://doi. org/10.1161/HYPERTENSIONAHA.109.148635.
[30] Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, et al. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275:29717–23. https://doi.org/10.1074/jbc.M003128200.
[31] Chou CH, Hung CS, Liao CW, Wei LH, Chen CW, Shun CT, et al. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc Res. 2018;114:690–702. https://doi.org/ 10.1093/cvr/cvy013.
[32] Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J Biol Chem. 2012;287: 2666–77. https://doi.org/10.1074/jbc.M111.246173.
[33] Sackett SD, Otto T, Mohs A, Sander LE, Strauch S, Streetz KL, et al. Myeloid cells require gp130 signaling for protective antiinflammatory functions during sepsis. FASEB J. 2019;33:6035– 44. https://doi.org/10.1096/fj.201802118R.
[34] Yokoe I, Kobayashi H, Kobayashi Y, Giles JT, Yoneyama K, Kitamura N, et al. Impact of tocilizumab on N-terminal pro-brain natriuretic peptide levels in patients with active rheumatoid arthritis without cardiac symptoms. Scand J Rheumatol. 2018;47:364– 70. https://doi.org/10.1080/03009742.2017.1418424.
Rights and Permissions
This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. You are not required to obtain permission to reuse this article. To request permission for a type of use not listed, please contact Biotechnology Kiosk.